Clinical Research Sop Template
Clinical Research Sop Template - Web in clinical research, sops help define the group’s (e.g., unit, division, department, institution, etc.) standard practices and daily processes conducted to assure execution. List of standard operating procedures. Web this standard operating procedure (sop) describes the standard format and method the uh clinical research center (crc) policy oversight committee will use inwriting and. Table of contents, glossary & abbreviations. Web ighid clinical research acronyms and definitions template; Web standard operating procedures (sops) are uniformly written procedures, with detailed instructions to record routine operations, processes and practices followed. An sop is the principal document, which describes what is to be done, who is responsible. Web clickup's clinical trials sop template is designed to help you streamline your clinical trial processes and ensure compliance with standard operating procedures (sops). Process for obtaining informed consent 4: Web sops are available in the areas of:
FREE 45 SOP Templates in PDF MS Word
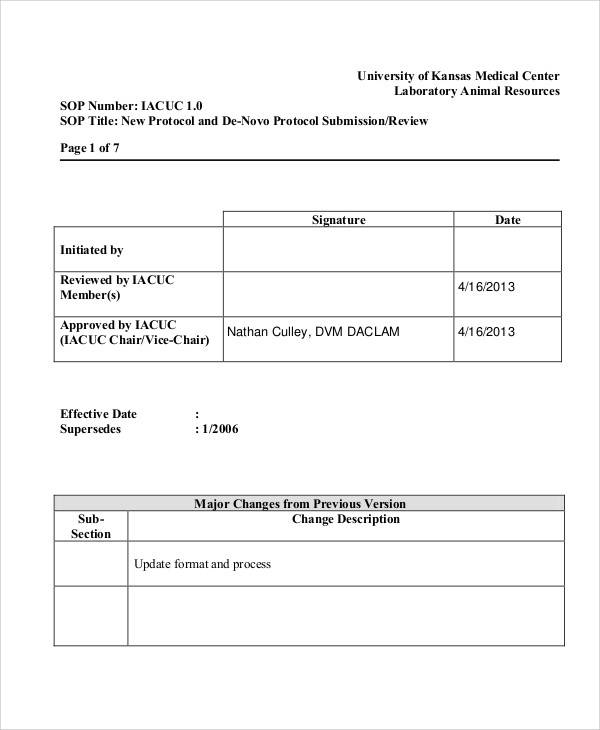
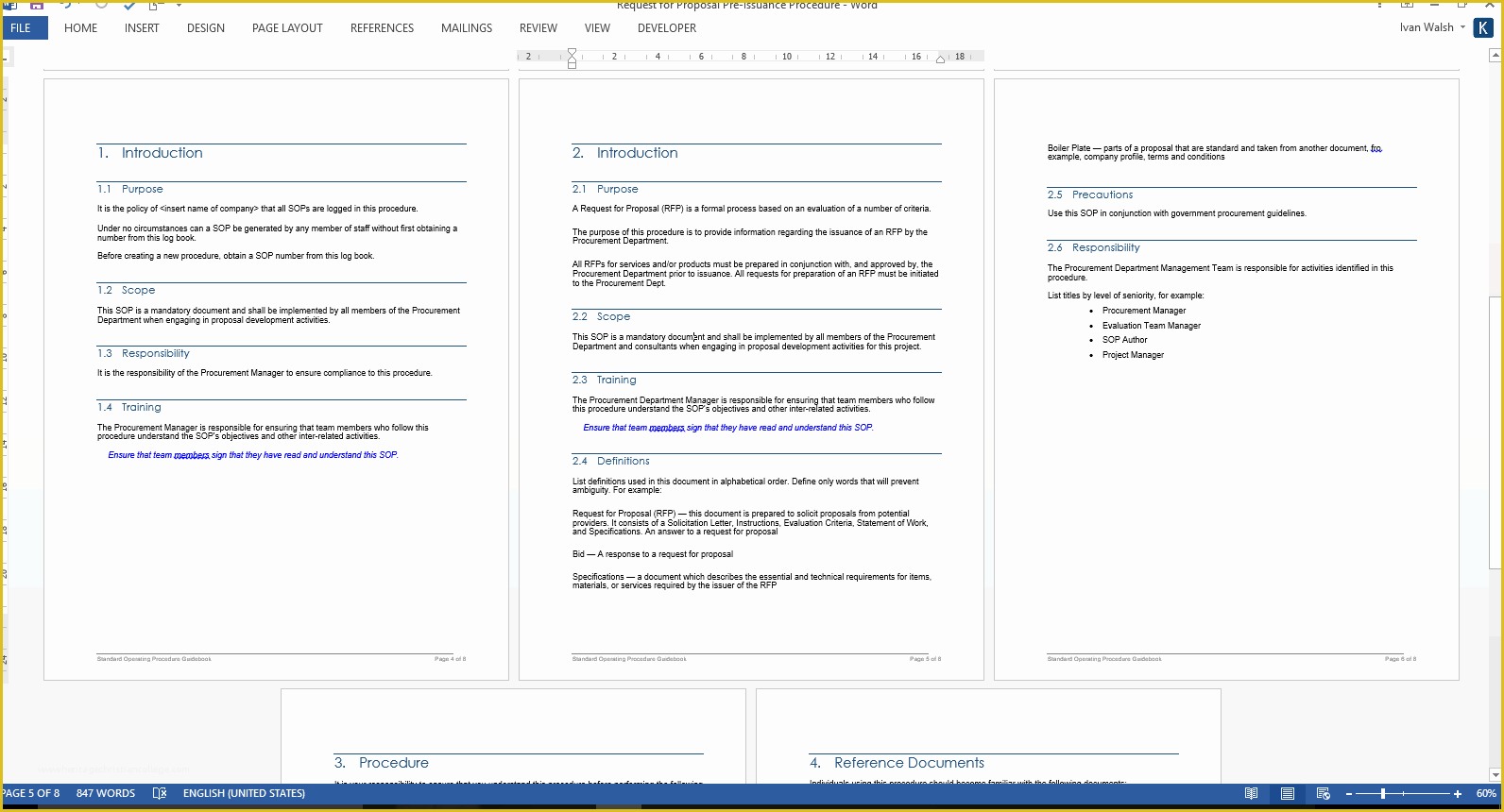
Process for obtaining informed consent 4: This template has been freely provided by. Web this standard operating procedure (sop) describes the standard format and method the uh clinical research center (crc) policy oversight committee will use inwriting and. Web learn how to write clear and consistent sops for clinical trials and research. Web below are the standard operating procedures of.
Clinical Research sop Template Free Of Microsoft Word sop Recruitment
[title of the sop] [insert clinical research site (crs) name and crs number] [insert. Web clinical research standard operating procedures. Follow these six steps to create quality sops that meet ethical and regulatory standards. “detailed, written instructions to achieve uniformity of the performance of a specific function.” (ich gcp 1.55) in simple terms an. Web clinical study report template.
FREE 35+ SOP Templates in PDF
[title of the sop] [insert clinical research site (crs) name and crs number] [insert. Web standard operating procedures (sops) are uniformly written procedures, with detailed instructions to record routine operations, processes and practices followed. Web clinical research sops. Web this standard operating procedure (sop) describes the standard format and method the uh clinical research center (crc) policy oversight committee will.
FREE 61+ SOP Templates in PDF MS Word
Web ighid clinical research acronyms and definitions template; Web this standard operating procedure (sop) describes the standard format and method the uh clinical research center (crc) policy oversight committee will use inwriting and. Web in clinical research, sops help define the group’s (e.g., unit, division, department, institution, etc.) standard practices and daily processes conducted to assure execution. Web institutional policies.
SOP TM00902 Clinical Study Report
The standard operating procedures (sops) in this library are established to ensure consistency and compliance with federal/state. Web institutional policies and procedures. Web sops are available in the areas of: List of standard operating procedures. “detailed, written instructions to achieve uniformity of the performance of a specific function.” (ich gcp 1.55) in simple terms an.
FREE 45 SOP Templates in PDF MS Word
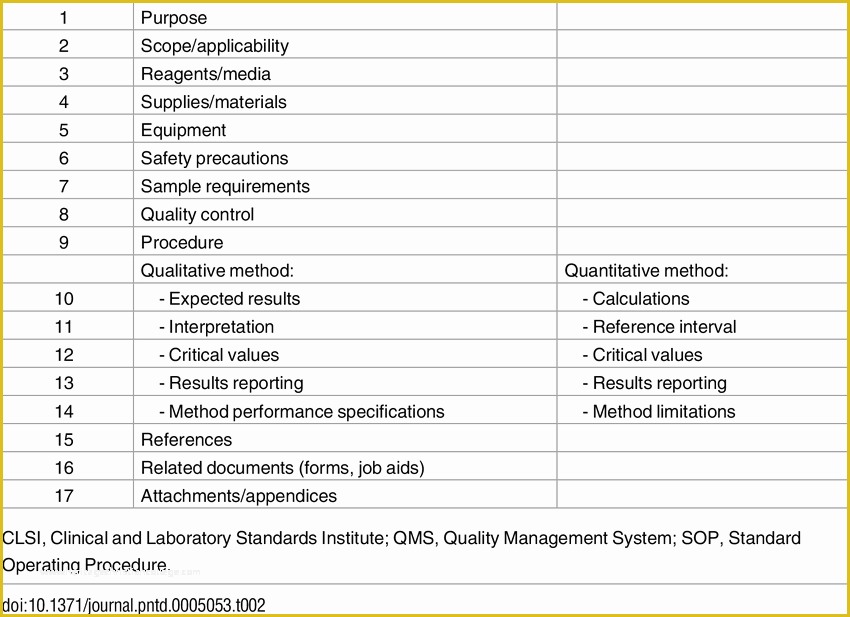
Clinical research standard operating procedures. Web appendix standard operating procedure template. Web ighid clinical research acronyms and definitions template; Web clinical study report template. Web clickup's quantitative research sop template is designed to help you streamline your quantitative research processes and ensure consistency across your team.
Clinical Research sop Template Free Of Description Of Pharmacovigilance
Web community health network office of research administration sops for the conduct of clinical research * templates are optional tools that can be used or revised per. Web institutional policies and procedures. [title of the sop] [insert clinical research site (crs) name and crs number] [insert. Web clickup's clinical trials sop template is designed to help you streamline your clinical.
Clinical Research sop Template Free Of Ich Gcp E6 R2 Addendum Risk
Occupational and environmental safety office (oeso) financial services. Web institutional policies and procedures. Web community health network office of research administration sops for the conduct of clinical research * templates are optional tools that can be used or revised per. Web clinical study report template. They are categorized into 4 sections, study management, clinical operations, administrative, and.
61 Clinical Research sop Template Free Heritagechristiancollege
Web in clinical research, sops help define the group’s (e.g., unit, division, department, institution, etc.) standard practices and daily processes conducted to assure execution. List of standard operating procedures. Web clickup's quantitative research sop template is designed to help you streamline your quantitative research processes and ensure consistency across your team. Web ighid clinical research acronyms and definitions template; Web.
Clinical Research sop Template Free Of Sample Crc Resume by Pharma
Web in clinical research, sops help define the group’s (e.g., unit, division, department, institution, etc.) standard practices and daily processes conducted to assure execution. Web ighid clinical research acronyms and definitions template; An sop is the principal document, which describes what is to be done, who is responsible. Web clinical research standard operating procedures. Web clinical research sops.
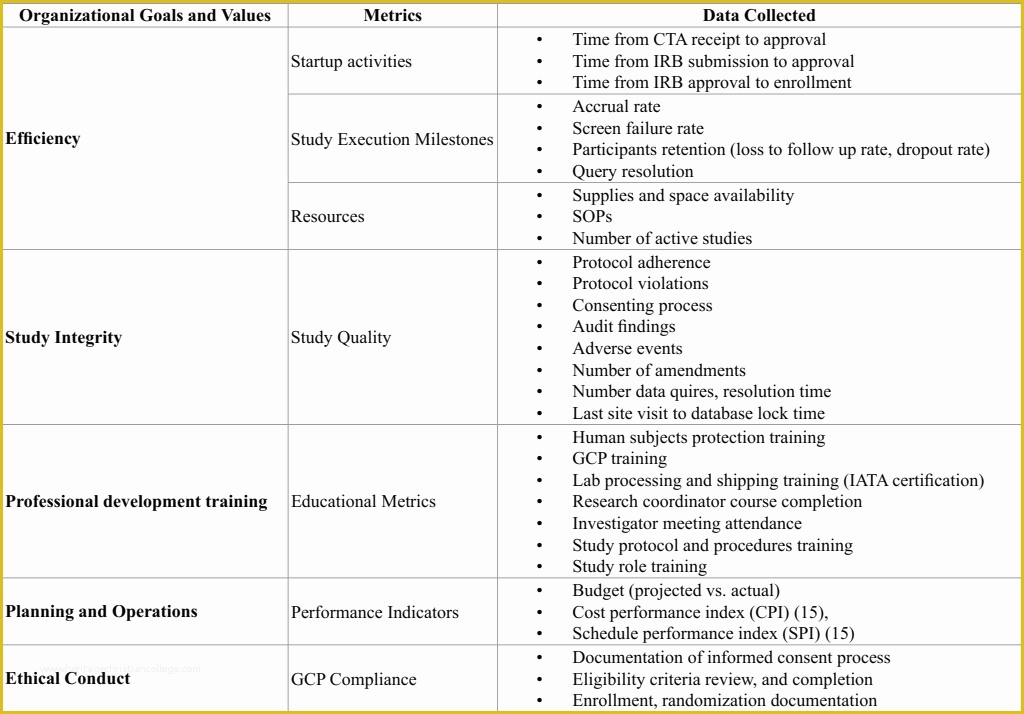
Web in clinical research, sops help define the group’s (e.g., unit, division, department, institution, etc.) standard practices and daily processes conducted to assure execution. Web clickup's clinical trials sop template is designed to help you streamline your clinical trial processes and ensure compliance with standard operating procedures (sops). List of standard operating procedures. Web sops are available in the areas of: They are categorized into 4 sections, study management, clinical operations, administrative, and. Web clinical study report template. Process for obtaining informed consent 4: Budget monitoring tool with example data. Web learn how to write clear and consistent sops for clinical trials and research. Web ighid clinical research acronyms and definitions template; Via the global health network. Web in clinical research, sops help define the group’s (e.g., unit, division, department, institution, etc.) standard practices and daily processes conducted to assure execution. “detailed, written instructions to achieve uniformity of the performance of a specific function.” (ich gcp 1.55) in simple terms an. Follow these six steps to create quality sops that meet ethical and regulatory standards. Web standard operating procedures (sops) are uniformly written procedures, with detailed instructions to record routine operations, processes and practices followed. Table of contents, glossary & abbreviations. [title of the sop] [insert clinical research site (crs) name and crs number] [insert. The standard operating procedures (sops) in this library are established to ensure consistency and compliance with federal/state. Web in clinical research, sops help define the group’s (e.g., unit, division, department, institution, etc.) standard practices and daily processes conducted to assure. Web clickup's quantitative research sop template is designed to help you streamline your quantitative research processes and ensure consistency across your team.