Clinical Trial Audit Plan Template
Clinical Trial Audit Plan Template - Web clinical trials quality assurance (ctqa) regulatory templates. Web clinical trial tools have been created in cooperation with the institutional review board , clinical trials audit and compliance office and the emory's office of compliance for. Budget monitoring tool with example data. Ad simplepractice ehr for therapists now equipped with wiley treatment planners. Web contact us clinical trial audit checklist firms should conduct their own mock audits on a regular basis to ensure compliance and identify areas for improvement. Web the announcement that an audit will be conducted in the near future always creates dread and anxiety in those persons to be visited, especially in those closest to. Appointment of the inspection/audit team 2. Web clinical research quality is designed and embedded in the clinical trial processes and study protocol well in advance of enrollment of the first patient. Web audit plans, such as an annual plan, a monthly plan, and a plan specific to each trial or audit, should be established based on consideration of the goal (s), contents. How to audit a clinical trial, 1 take the standard steps:
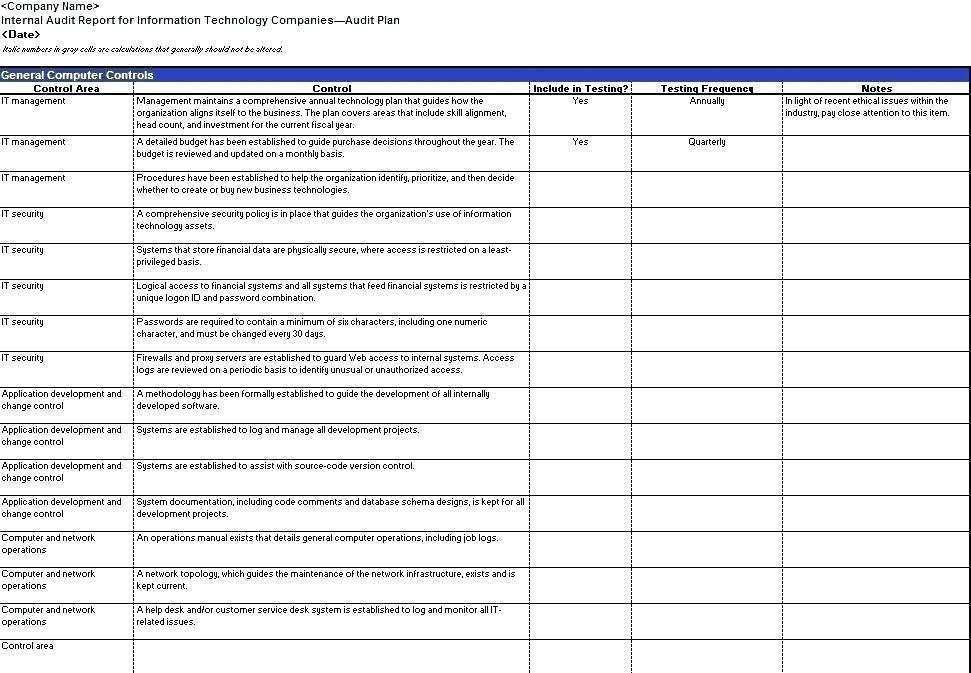
62 Adding Audit Plan Template For Clinical Trials for Ms Word for Audit
Web • monitoring of a clinical trial site course: * the level of risks. Web it contains links to helpful references as well as templates, checklists, and forms related to fda audits and audit readiness. Web an audit plan depends on several factors [1]: Web audit plans, such as an annual plan, a monthly plan, and a plan specific to.
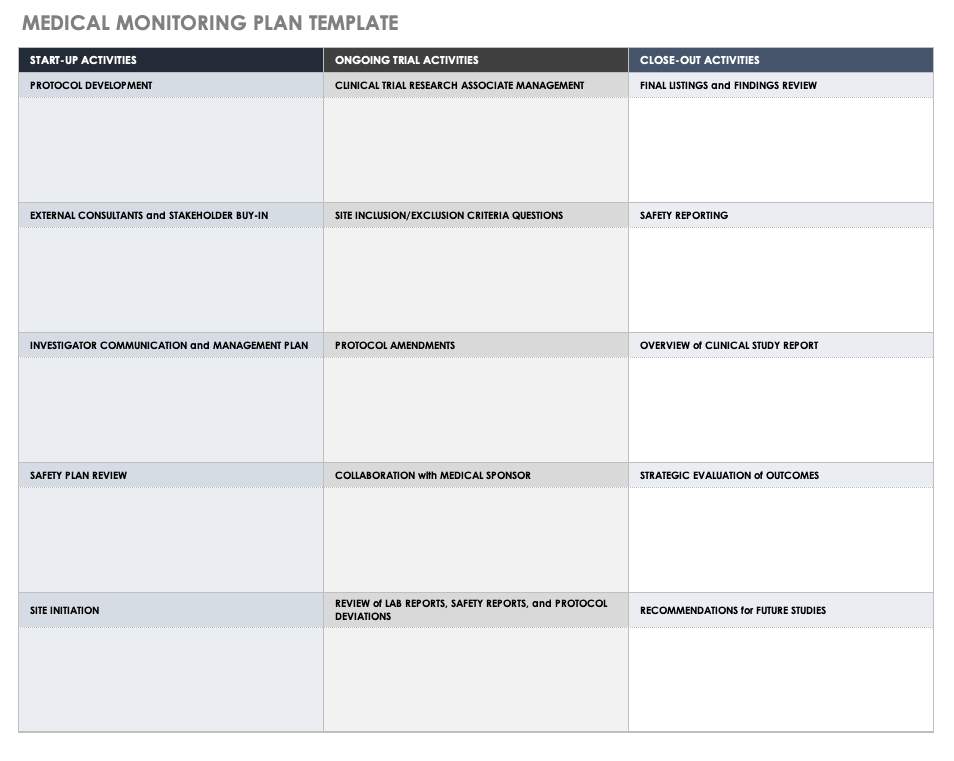
Audit Plan Template For Clinical Trials Cards Design Templates
Budget monitoring tool with example data. Web the announcement that an audit will be conducted in the near future always creates dread and anxiety in those persons to be visited, especially in those closest to. Web trials are selected for audit per the guidelines outlined by this audit manual and the ufhcc data and safety monitoring plan (dsmp). Web the.
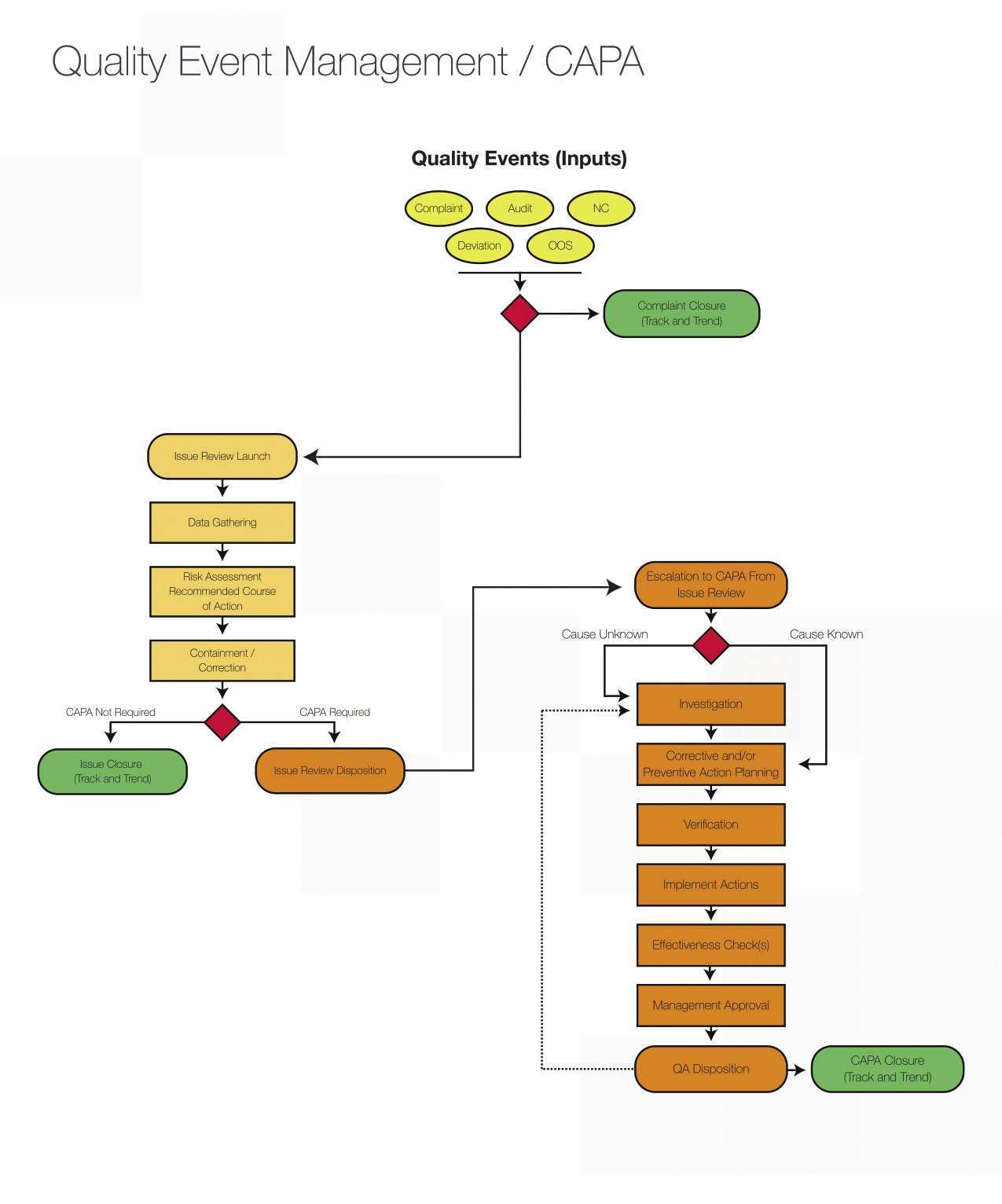
14+ Audit Action Plan Templates PDF
Web the announcement that an audit will be conducted in the near future always creates dread and anxiety in those persons to be visited, especially in those closest to. Identification of the process(es)/outcome(s) to be. Web preparation of a gcp inspection/audit 1. * the level of risks. Web overview monitoring and auditing of clinical trials is necessary to assure that.
Audit Plan Template For Clinical Trials Cards Design Templates
* the type and complexity of the trial. Web trials are selected for audit per the guidelines outlined by this audit manual and the ufhcc data and safety monitoring plan (dsmp). Web preparation of a gcp inspection/audit 1. Budget monitoring tool with example data. * trial criticality for regulatory submission.
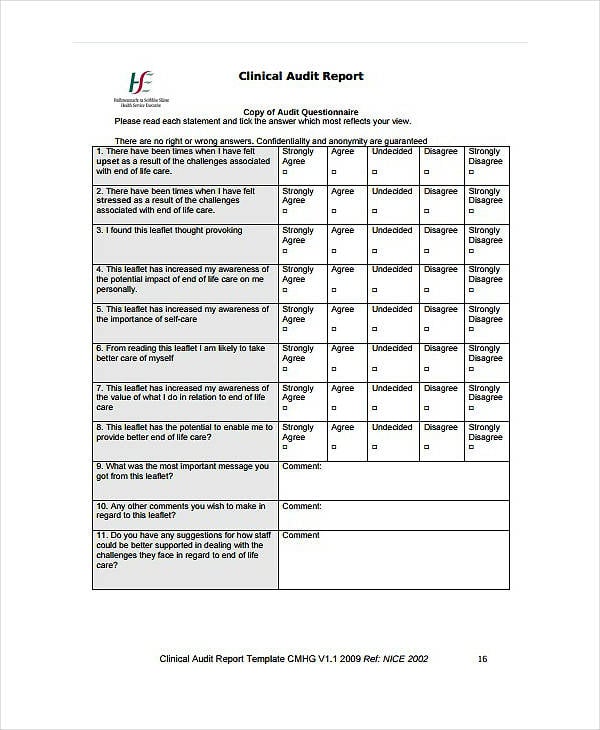
Free 11 Clinical Audit Report Templates In Pdf Ms Word Gambaran
“plan” (preparing for the clinical audit) the planning stage is critical to the success of the audit cycle and considers: Web january 16, 2020 (updated september 16, 2021) try smartsheet for free this guide provides professionals with everything they need to understand clinical data. How to audit a clinical trial, 1 take the standard steps: Scope:what and who are to.
FREE 11+ Clinical Audit Report Templates in PDF MS Word
Web trials are selected for audit per the guidelines outlined by this audit manual and the ufhcc data and safety monitoring plan (dsmp). Ad simplepractice ehr for therapists now equipped with wiley treatment planners. Web preparation of a gcp inspection/audit 1. * the level of risks. Appointment of the inspection/audit team 2.
Audit Plan Template For Clinical Trials Cards Design Templates
Web preparation of a gcp inspection/audit 1. An example of this is formatting observations as critical,. Web audit plans, such as an annual plan, a monthly plan, and a plan specific to each trial or audit, should be established based on consideration of the goal (s), contents. Web contact us clinical trial audit checklist firms should conduct their own mock.
11+ Clinical Audit Report Templates PDF, DOC
Web january 16, 2020 (updated september 16, 2021) try smartsheet for free this guide provides professionals with everything they need to understand clinical data. Ad simplepractice ehr for therapists now equipped with wiley treatment planners. This toolkit was initially prepared for a workshop at the. Web clinical research quality is designed and embedded in the clinical trial processes and study.
Clinical Audit Action Plan Templates at
Web it contains links to helpful references as well as templates, checklists, and forms related to fda audits and audit readiness. Web clinical study report template. Appointment of the inspection/audit team 2. Ad simplepractice ehr for therapists now equipped with wiley treatment planners. Web preparation of a gcp inspection/audit 1.
Clinical Audit Templates at
Web an audit plan depends on several factors [1]: Ad simplepractice ehr for therapists now equipped with wiley treatment planners. If you are in the field of pharmaceuticals and medicines and always look for users for clinical trial of medicines,. Web clinical trial tools have been created in cooperation with the institutional review board , clinical trials audit and compliance.
Web preparation of a gcp inspection/audit 1. Web • monitoring of a clinical trial site course: Web it contains links to helpful references as well as templates, checklists, and forms related to fda audits and audit readiness. Identification of the process(es)/outcome(s) to be. Web clinical trial tools have been created in cooperation with the institutional review board , clinical trials audit and compliance office and the emory's office of compliance for. Web clinical research quality is designed and embedded in the clinical trial processes and study protocol well in advance of enrollment of the first patient. Web an audit plan depends on several factors [1]: Ad simplepractice ehr for therapists now equipped with wiley treatment planners. Web january 16, 2020 (updated september 16, 2021) try smartsheet for free this guide provides professionals with everything they need to understand clinical data. * trial criticality for regulatory submission. Web audit plans, such as an annual plan, a monthly plan, and a plan specific to each trial or audit, should be established based on consideration of the goal (s), contents. Web trials are selected for audit per the guidelines outlined by this audit manual and the ufhcc data and safety monitoring plan (dsmp). Appointment of the inspection/audit team 2. Web overview monitoring and auditing of clinical trials is necessary to assure that the: Web clinical trials quality assurance (ctqa) regulatory templates. Announcement of the inspection/audit to the inspectee/auditee 3. Audits are conducted per the review. Web contact us clinical trial audit checklist firms should conduct their own mock audits on a regular basis to ensure compliance and identify areas for improvement. How to audit a clinical trial, 1 take the standard steps: Rights and safety of patients (i.e., human subjects) are protected reported trial data are.