Device History Record Template
Device History Record Template - Simply because, it is unique to your device and system. Web device history records contain the complete history of every medical device that your medical devices company manufactures. Web the “device history record”. Web what is a device history record (dhr)? (d) the acceptance records which demonstrate the device is. Web (a) the dates of manufacture; The device history record (dhr) is outlined in the us fda quality system requirements, part 820, section 184. The history and information related to how you made the device, in accordance. (c) the quantity released for distribution; Web it’s also helpful to your children and their children, further down the road of life.
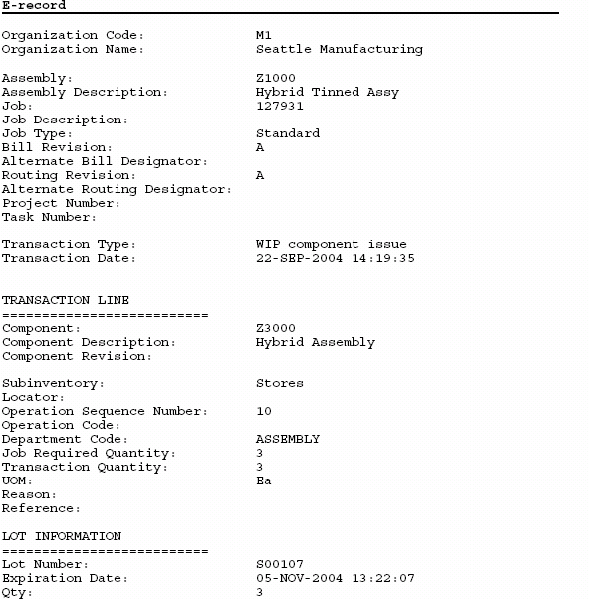
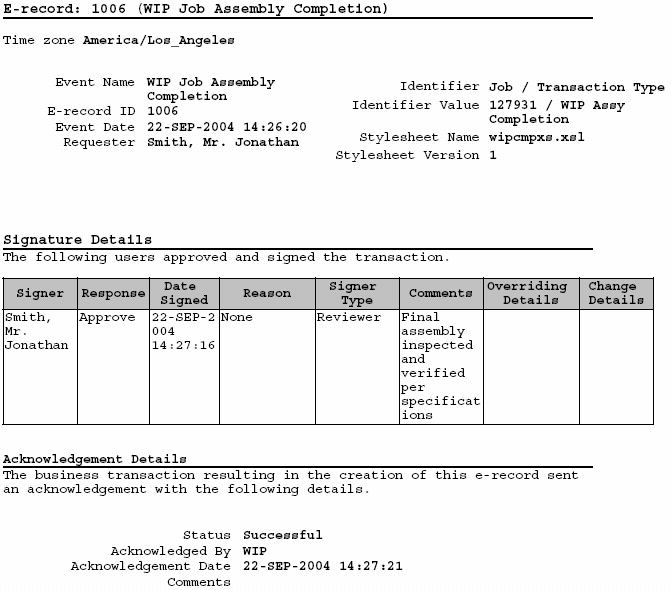
Oracle Manufacturing Implementing Oracle ERecords in Discrete
By using medical device qms. Web a device history record (dhr) refers to a compilation of records containing the production history of a finished device and is defined under subpart m 21 cfr part 820 (section. Device account records (dhrs) are ampere crucial portion of the medizintechnik device quality management system. Web think of it this way: Web it’s also.
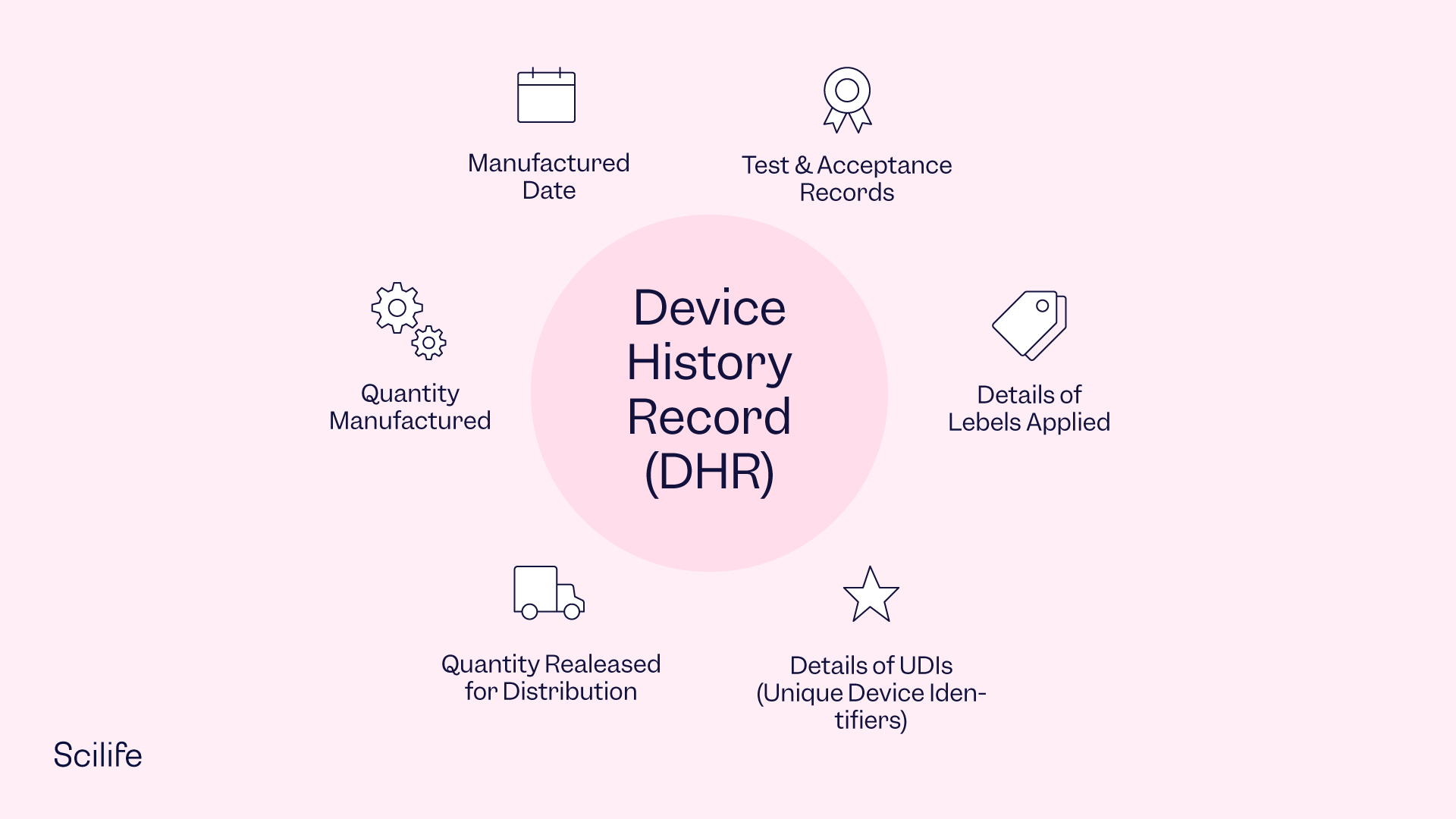
What is Device History Record (DHR)? Complete definition Scilife
Simply because, it is unique to your device and system. Web for starters, you’ll need a digital master template and device history record, allowing you to review, approve and complete a master template to create edhr. Web what is a device history record (dhr)? Device history record (dhr) means a compilation of records containing the production. Identify key definitions related.
Device Master Record Index Template
Web in medical device and diagnostic manufacturing, companies must keep a complete and accurate record of each product they produce in the form of a device. Device history record dhfs for software in medical devices. The fda mandates that every. The device history record (dhr) is outlined in the us fda quality system requirements, part 820, section 184. Describe requirements.
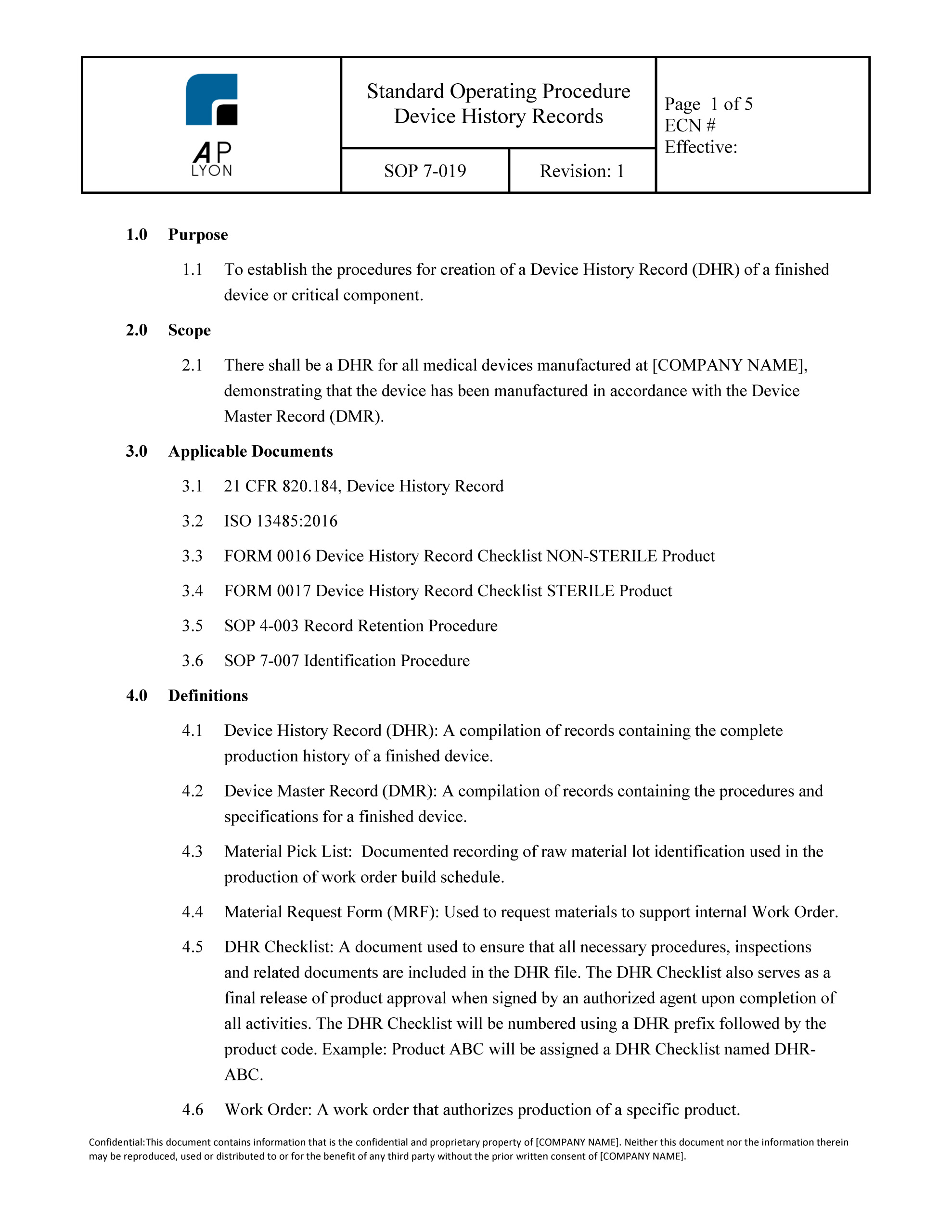
Device History Record Procedure
The device history record is literally the history of the device. Web the fully documentation about the manufacturing or tracking of every medical device that your company sold is contained in a device history record (dhr). (c) the quantity released for distribution; Web (a) the dates of manufacture; The essential components of a dhf design history file vs.
Oracle Manufacturing Implementing Oracle ERecords in Discrete
(c) the quantity released for distribution; Web in medical device and diagnostic manufacturing, companies must keep a complete and accurate record of each product they produce in the form of a device. This appendix covers the following. Simply because, it is unique to your device and system. (d) the acceptance records which demonstrate the device is.
Federal Register Unique Device Identification System
Web what is a device history record (dhr)? Web (a) the dates of manufacture; (d) the acceptance records which demonstrate the device is. Web in medical device and diagnostic manufacturing, companies must keep a complete and accurate record of each product they produce in the form of a device. Web think of it this way:
PPT Design documentation PowerPoint Presentation ID1625484
Web device history records contain the complete history of every medical device that your medical devices company manufactures. This appendix covers the following. The free family history records is a template that helps you organize the important information. Us food and drug administration’s (us. By using medical device qms.
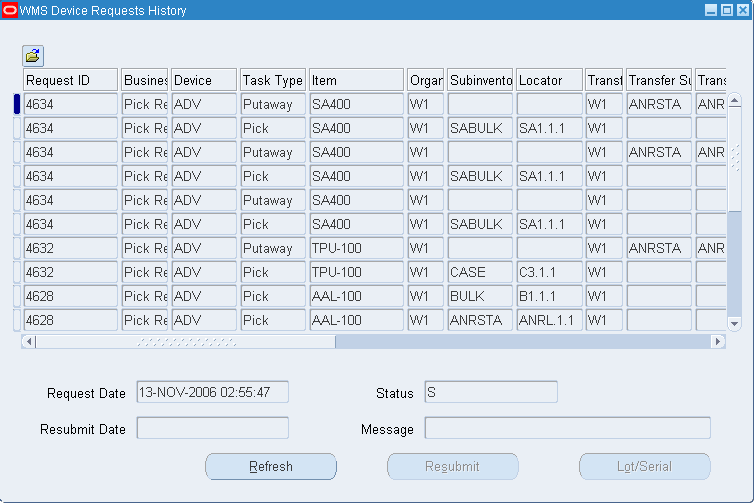
Oracle Warehouse Management User's Guide
Web a device history record (dhr) refers to a compilation of records containing the production history of a finished device and is defined under subpart m 21 cfr part 820 (section. Web device history record shall be defined as the compilation of records containing the complete production / maintenance history of a finished product and showing latest. Device account records.
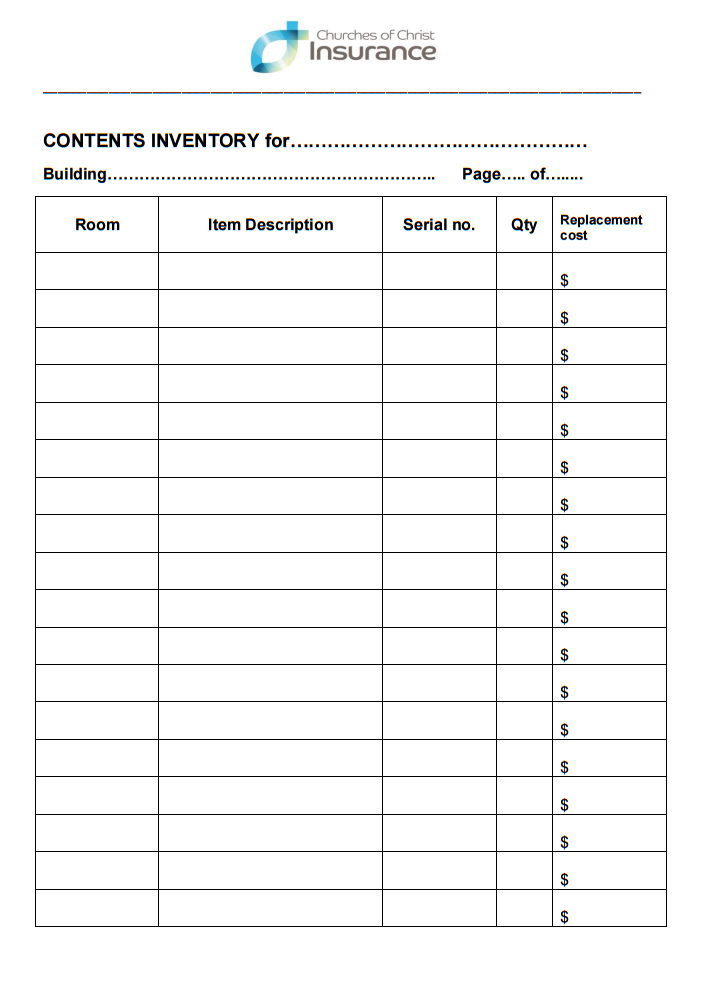
Why You Should Keep a Record of Device Serial Numbers Churches of
21 cfr 820.3 (i) provides the following definition: The essential components of a dhf design history file vs. Web mdf record book template. [definition and components] read below if you would like more information regarding device history records and its. Web device history records contain the complete history of every medical device that your medical devices company manufactures.
Device Master Records & Design History Files
Web the device history record (dhr) demonstrates that all batch, lot, or manufacturing unit in a pharmaceutical device was manufactured according to the specifications in the device. By using medical device qms. Us food and drug administration’s (us. Web device history record shall be defined as the compilation of records containing the complete production / maintenance history of a finished.
The fda mandates that every. Web what is a device history record (dhr)? Web the device history record (dhr) demonstrates that all batch, lot, or manufacturing unit in a pharmaceutical device was manufactured according to the specifications in the device. Specifically, the dhr shall include: The specific contents of the device history record are reported within 21 cfr 820.184. Web a device history record (dhr) refers to a compilation of records containing the production history of a finished device and is defined under subpart m 21 cfr part 820 (section. The essential components of a dhf design history file vs. Web in medical device and diagnostic manufacturing, companies must keep a complete and accurate record of each product they produce in the form of a device. (d) the acceptance records which demonstrate the device is. Describe requirements and intent for document controls,. (d) the acceptance records which demonstrate the device is. Web the fully documentation about the manufacturing or tracking of every medical device that your company sold is contained in a device history record (dhr). The free family history records is a template that helps you organize the important information. Web device history records contain the complete history of every medical device that your medical devices company manufactures. Web the “device history record”. Web for starters, you’ll need a digital master template and device history record, allowing you to review, approve and complete a master template to create edhr. By using medical device qms. Web what is a design history file? (a) the dates of manufacture; Web fyi, there is no such thing as a dhr template.