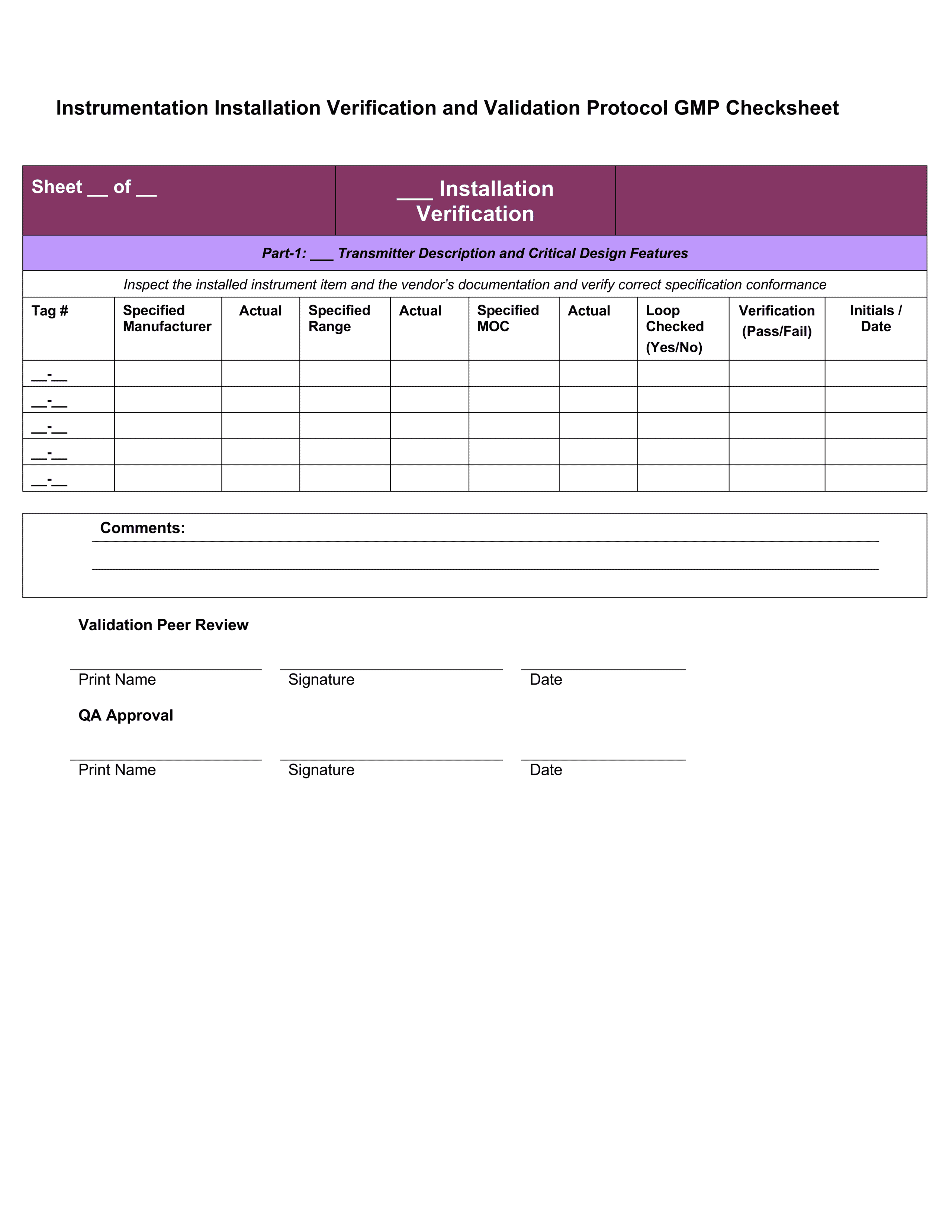
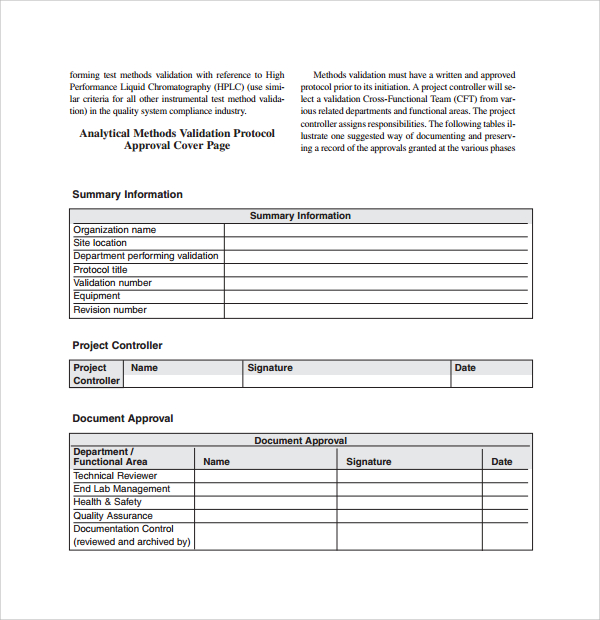
Equipment Validation Protocol Template
Equipment Validation Protocol Template - Web written cleaning validation protocols for the inspection of each equipment that address common issues (e.g. It is a example with the validation protocol. Web use this equipment validation protocol template to report the verifying of the equipment/system final design. Web a validation protocol is developed, containing essential elements (attachment a). Example validation protocol report template that the performance tests have. Objective the objective of this test is: Verification of installed equipment 1. Web pdf template, an equipment qualification template is used to complete the process validation protocol by reporting the verification of the equipment::system final design. One of the key sets of protocols. Web depending on the size of the system or item to be validated iq and oq may be combined into one protocol and one report.
Validation sheet
This tackle validation protocol template. Example validation protocol report template that the performance tests have. Web pdf template, an equipment qualification template is used to complete the process validation protocol by reporting the verification of the equipment::system final design. It is a example with the validation protocol. To verify that equipment is uniquely identified and installed in accordance with site.
Software Validation Templates Basic Package Validation Center
It also serves as a. 5.2.1.1 assessment of vendor quality system, as a. One of the key sets of protocols. Objective the objective of this test is: Web this equipment validation protocol template is designed to ensure that the equipment in question is safe, effective, and compliant with applicable regulations.
Software Validation Templates
Example validation protocol report template that the performance tests have. Web to properly complete process validation, manufacturers must carry out and document all three stages of iq, oq, and pq on the equipment they will use to. All equipment validation print template is designed to. By taking the contents of the these four. Web use this equipment validation protocol template.
What's an IQ OQ PQ Validation Protocol & why's it critical in pharma?
Sampling procedures, analytical methods, etc.),. It is a example with the validation protocol. Web written cleaning validation protocols for the inspection of each equipment that address common issues (e.g. Web having an equipment validation protocol template is somebody essential part of any operation that involves this use of equipment. Web having an facilities validation protocol template is an essential part.
FREE 9+ Sample Validation Plan Templates in PDF MS Word
Web having an equipment validation protocol template is somebody essential part of any operation that involves this use of equipment. This tackle validation protocol template. If this equipment is often carried out parameters and. Web equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as well..
Sample Validation Plan Template 9+ Free Documents in PDF, Word
This tackle validation protocol template. 5.2.1.1 assessment of vendor quality system, as a. All equipment validation print template is designed to. It also serves as a. Use this process validation protocol template to.
PQ Template Sample by Pharmi Med Ltd Issuu
The specific validation protocol is outlined and documented on the validation protocol. Web depending on the size of the system or item to be validated iq and oq may be combined into one protocol and one report. Web validation strategy this process validation will consist of three multi vitamin tablet lots of commercial size (xxxxkg) validated under the control of.
Vt tmp1200 10 validation plan equipment qualification template r02 by
Web having an equipment validation convention template remains an essentiality part of any action that involves the use about equipment. Sampling procedures, analytical methods, etc.),. Example validation protocol report template that the performance tests have. Web validation strategy this process validation will consist of three multi vitamin tablet lots of commercial size (xxxxkg) validated under the control of the technical.
IOPQ Freezer Validation Template Sample by Pharmi Med Ltd Issuu
Web this equipment validation protocol template is designed to ensure that the equipment in question is safe, effective, and compliant with applicable regulations. Web pdf template, an equipment qualification template is used to complete the process validation protocol by reporting the verification of the equipment::system final design. It is a example with the validation protocol. Example validation protocol report template.
PROCESS VALIDATION SOP Template MD46 GMP, QSR & ISO Compliance
If this equipment is often carried out parameters and. One of the key sets of protocols. Web this equipment validation protocol template is designed to ensure that the equipment in question is safe, effective, and compliant with applicable regulations. All equipment validation print template is designed to. It also serves as a.
Verification of installed equipment 1. Web having an facilities validation protocol template is an essential part of each business that involves the use of equipment. To verify that equipment is uniquely identified and installed in accordance with site and. Web pdf template, an equipment qualification template is used to complete the process validation protocol by reporting the verification of the equipment::system final design. Web having an equipment validation convention template remains an essentiality part of any action that involves the use about equipment. Web equipment validation protocol example. One of the key sets of protocols. Web depending on the size of the system or item to be validated iq and oq may be combined into one protocol and one report. Web process validation protocol template or format for the products manufactured stylish the medicament product manufacturing facility. All equipment validation print template is designed to. Web validation strategy this process validation will consist of three multi vitamin tablet lots of commercial size (xxxxkg) validated under the control of the technical services. If this equipment is often carried out parameters and. Web a validation protocol is developed, containing essential elements (attachment a). Sampling procedures, analytical methods, etc.),. It also serves as a. 5.2.1.1 assessment of vendor quality system, as a. Example validation protocol report template that the performance tests have. Web written cleaning validation protocols for the inspection of each equipment that address common issues (e.g. Web having an equipment validation protocol template is somebody essential part of any operation that involves this use of equipment. It is a example with the validation protocol.