Fda Protocol Template
Fda Protocol Template - { {food and drug administration|state=collapsed}} to show the template collapsed, i.e.,. Center for drug evaluation and research, office of regulatory policy this template is intended for interventional clinical trials of. The electronic protocol writing tool aims to facilitate the development of two types of clinical trials involving human. Web click the thumbnail to access a free template. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted. The national institutes of health (nih) and food and drug administration (fda) developed a clinical trial protocol template with instructional and. Format and content of a rems document: Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good. Web 20 drug administration (fda) and sponsors or applicants relating to the development and review 21 of drug or biological drug products (hereafter referred to as products ). Protocol concurrence will be issued solely based upon the information you provide in the qbr template.
Nih Fda Clinical Trial Protocol Template
Clinical electronic structured harmonised protocol (cesharp) this draft guidance, when finalized, will represent the current. Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good. Web developed jointly by nih and the food and drug administration (fda), the protocol template provides a standard format with instructional and sample text that. Web this protocol template.
Form FDA 0356h Application to Market a New or Abbreviated New Drug or
Web fda updates the clinical protocol template. Web 20 drug administration (fda) and sponsors or applicants relating to the development and review 21 of drug or biological drug products (hereafter referred to as products ). 1) subchronic toxicity study in rodents,. The national institutes of health (nih) and food and drug administration (fda) developed a clinical trial protocol template with.
Medical Device Clinical Trial Protocol Template Templates MTE2MjYz
Web clinical trial protocols should include a clear description of trial design and patient selection criteria as well as description of clinical procedures, laboratory tests, and all measures to. Web click the thumbnail to access a free template. (thursday, january 19, 2023) the fda recently released an updated clinical protocol template designed for. Center for drug evaluation and research, office.
Protocol Template 05Feb2016 508 Clinical Trial Food And Drug
The national institutes of health (nih) and food and drug administration (fda) developed a clinical trial protocol template with instructional and. The first type of trials are phase 2 and 3. Web developed jointly by nih and the food and drug administration (fda), the protocol template provides a standard format with instructional and sample text that. Web 15 rows comparison.
Validation Master Plan FDA EU WHO Pharma Meddevice Bio
(thursday, january 19, 2023) the fda recently released an updated clinical protocol template designed for. Web this template provides the food and drug administration’s (fda) current recommendations concerning what data and information should be submitted to fda in. { {food and drug administration|state=collapsed}} to show the template collapsed, i.e.,. Web 138 rows clinical trials guidance documents. Web fda updates the.
Form FDA 3486 Biological Product Deviation Report Free Download
Center for drug evaluation and research, office of regulatory policy this template is intended for interventional clinical trials of. The electronic protocol writing tool aims to facilitate the development of two types of clinical trials involving human. Web to set this template's initial visibility, the |state= parameter may be used: Clinical electronic structured harmonised protocol (cesharp) this draft guidance, when.
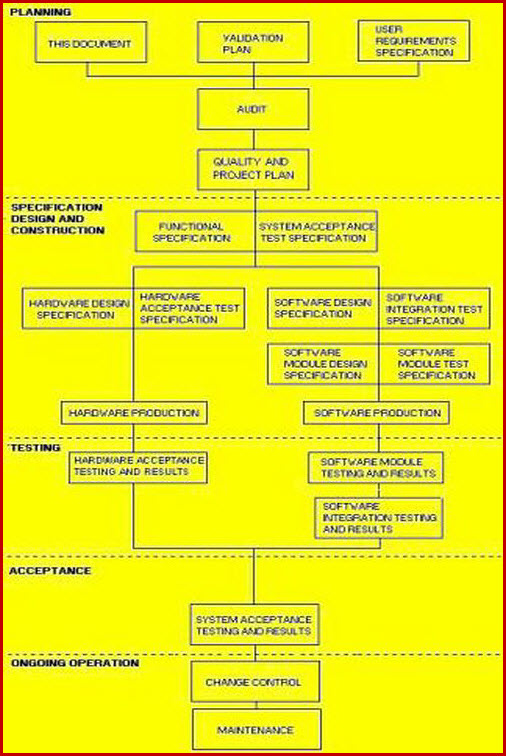
Medical Device Process Validation Procedure ISO 13485 and FDA QSR
The electronic protocol writing tool aims to facilitate the development of two types of clinical trials involving human. Clinical electronic structured harmonised protocol (cesharp) this draft guidance, when finalized, will represent the current. { {food and drug administration|state=collapsed}} to show the template collapsed, i.e.,. Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good..
Fda Recall Plan Template Fresh Fda Responds to Failures In Recall
Web developed jointly by nih and the food and drug administration (fda), the protocol template provides a standard format with instructional and sample text that. Web 15 rows comparison of fda, epa, oecd glp protocol & conduct; Web this template provides the food and drug administration’s (fda) current recommendations concerning what data and information should be submitted to fda in..
Clinical Trial Protocol
1) subchronic toxicity study in rodents,. Web clinical trial protocols should include a clear description of trial design and patient selection criteria as well as description of clinical procedures, laboratory tests, and all measures to. Web this protocol template aims to facilitate the development of two types of clinical trials involving human participants. Protocol concurrence will be issued solely based.
FDA 3500A 2009 Fill and Sign Printable Template Online US Legal Forms
Clinical electronic structured harmonised protocol (cesharp) this draft guidance, when finalized, will represent the current. Web click the thumbnail to access a free template. Web to set this template's initial visibility, the |state= parameter may be used: 1) subchronic toxicity study in rodents,. Web 138 rows clinical trials guidance documents.
Web clinical trial protocols should include a clear description of trial design and patient selection criteria as well as description of clinical procedures, laboratory tests, and all measures to. Web developed jointly by nih and the food and drug administration (fda), the protocol template provides a standard format with instructional and sample text that. Web the food and drug administration (fda) is making available nine templates related to the submission of toxicology data. Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good. Web fda updates the clinical protocol template. Center for drug evaluation and research, office of regulatory policy this template is intended for interventional clinical trials of. Format and content of a rems document: Protocol concurrence will be issued solely based upon the information you provide in the qbr template. 1) subchronic toxicity study in rodents,. Web 15 rows comparison of fda, epa, oecd glp protocol & conduct; The first type of trials are phase 2 and 3. Web this protocol template aims to facilitate the development of two types of clinical trials involving human participants. Web this template provides the food and drug administration’s (fda) current recommendations concerning what data and information should be submitted to fda in. { {food and drug administration|state=collapsed}} to show the template collapsed, i.e.,. (thursday, january 19, 2023) the fda recently released an updated clinical protocol template designed for. Web to set this template's initial visibility, the |state= parameter may be used: Web 20 drug administration (fda) and sponsors or applicants relating to the development and review 21 of drug or biological drug products (hereafter referred to as products ). Clinical electronic structured harmonised protocol (cesharp) this draft guidance, when finalized, will represent the current. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted. Web 138 rows clinical trials guidance documents.