Iso 13485 Software Validation Template
Iso 13485 Software Validation Template - Oliver eidel the iso 13485 is the standard for quality management in the medical device industry. Like us on google and comment here or. Oliver eidel template download this is a free template, provided by. Web this procedure is intended to meet the requirements of iso 13485:2016, clause 7.3.6 and 7.3.7 for design verification and design validation of medical device products. Like our facebook page and comment here or. Web this document applies to any software used in device design, testing, component acceptance, manufacturing, labelling, packaging, distribution and complaint handling or. Iso 13485 requirements are a great way to. Web updated june 9, 2022 template: Here you can check the complete list of documentation,. Web free iso 13485 software validation template.
Best Tips ISO 13485 procedures with our free template (Version 2016)
Like us on google and comment here or. Qmo continues to fill out the computerized system validation form by planning the validation anddocumenting the requirements for expected validation results. Web how to meet the software validation requirements of iso 13485:2016; Web templates iso 13485 templates updated june 9, 2022 template: Web validation 3.8.13 (bs en iso 9001:2015) confirmation, through the.
Free ISO 13485 Process Validation Template
Web this document applies to any software used in device design, testing, component acceptance, manufacturing, labelling, packaging, distribution and complaint handling or. Web you can buy the iso 13485 standard here. Oliver eidel template download this is a free template, provided by. Web free iso 13485 software validation template. Web updated june 9, 2022 template:
Iso 13485 Software Validation Template PDF Template
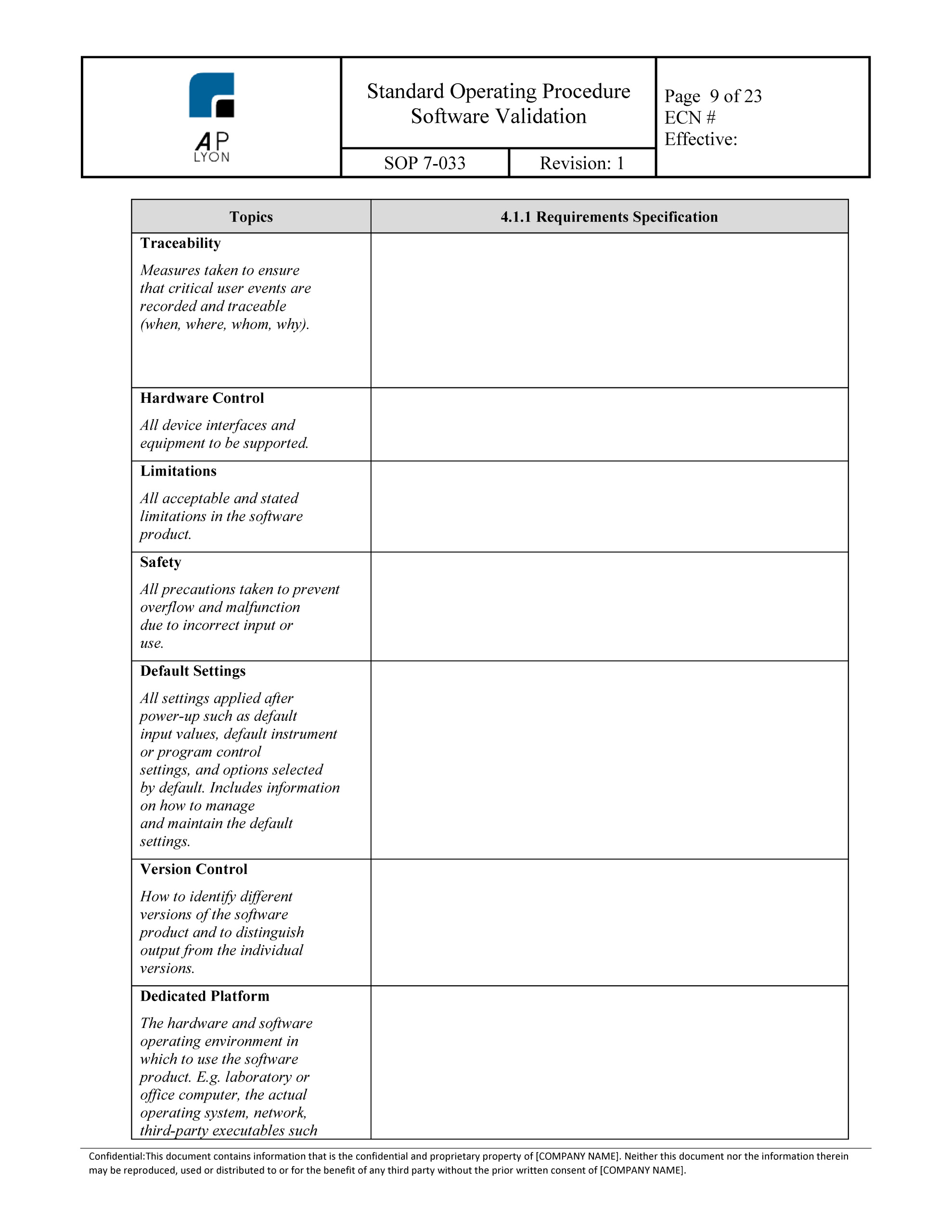
You can buy the iso 13485 standard here. Here you can check the complete list of documentation,. Qmo continues to fill out the computerized system validation form by planning the validation anddocumenting the requirements for expected validation results. Record of software validation the record provides information about software. Validate software which is used in the.
Software Validation Template Iso 13485
The main messages there are: Web this document applies to any software used in device design, testing, component acceptance, manufacturing, labelling, packaging, distribution and complaint handling or. Web free iso 13485 software validation template. Web our company is in the process of becoming iso 13485 compliant and as part of the quality management system, i have to come up with.
Software Validation Procedure
Web february 14, 2019 producing any part of a product includes validation and verification in its design and development. The main messages there are: Iso 13485 requirements are a great way to. Email us here from your work email (verifiable domain from company. Web validation of computer software is specified in section 4.1.6 of iso 13485:2016.
Publication of ISO134852016 Quality System Standard
Validate software which is used in the. Web validation 3.8.13 (bs en iso 9001:2015) confirmation, through the provision of objective evidence, that the requirements for a specific intended use or application have been. Web the documentation template may be used for iso 13485 certification audit purposes. Web our company is in the process of becoming iso 13485 compliant and as.
Software Validation Template Iso 13485
Email us here from your work email (verifiable domain from company. You can buy the iso 13485 standard here. Oliver eidel template download this is a free template, provided by. Web free iso 13485 software validation template. The main messages there are:
Iso 13485 2016 Templates Master of Documents
Like our facebook page and comment here or. The main messages there are: Here are all our posts on this. Document templates contain an average of twenty comments each,. Web this procedure is intended to meet the requirements of iso 13485:2016, clause 7.3.6 and 7.3.7 for design verification and design validation of medical device products.
Software Validation Procedure
Document templates contain an average of twenty comments each, and offer clear. Web templates iso 13485 templates updated june 9, 2022 template: A suggested layout of documenting risk within the master validation plan; Oliver eidel the iso 13485 is the standard for quality management in the medical device industry. Iso 13485 requirements are a great way to.
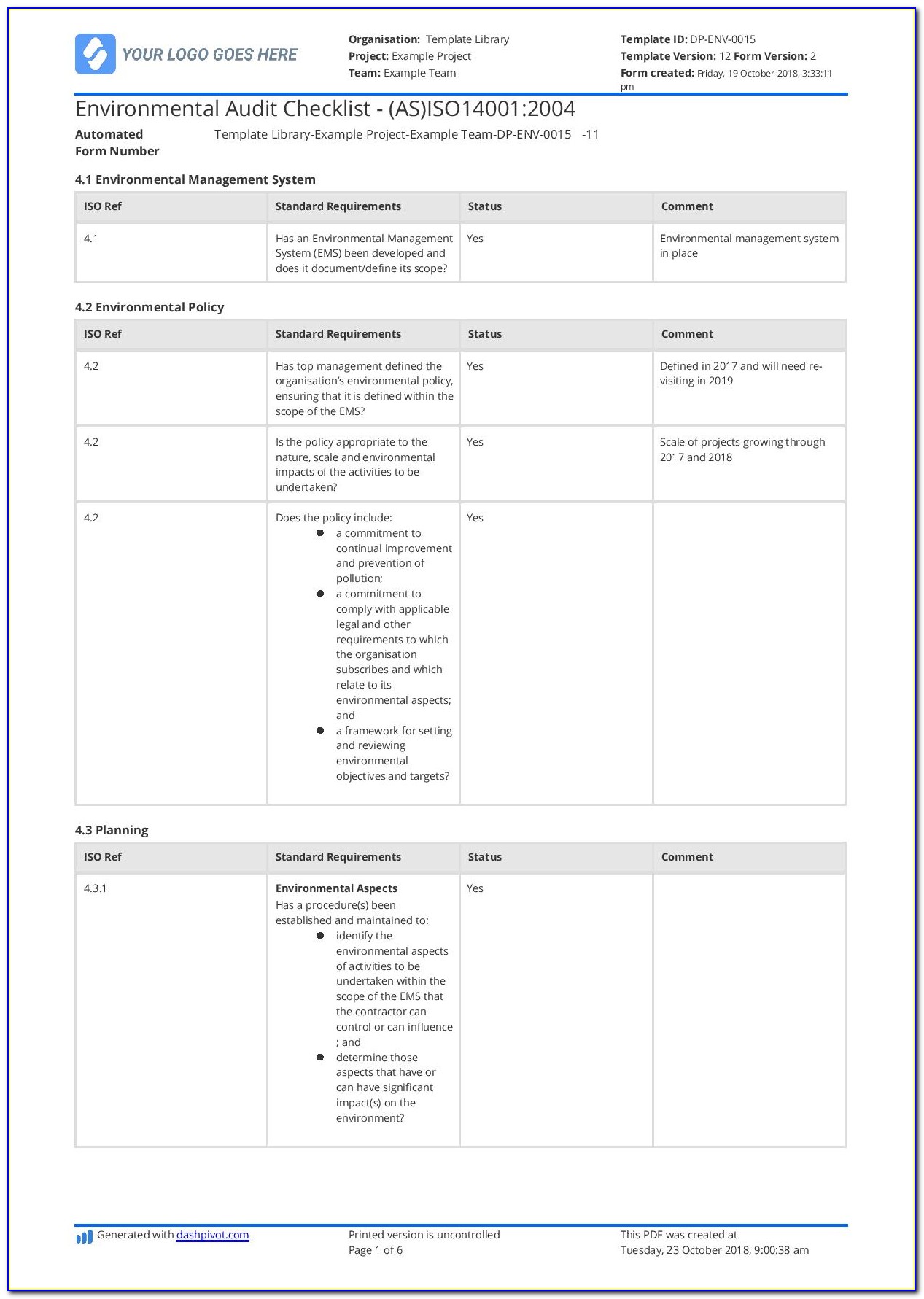
Iso 13485 Audit Report Template
Document templates contain an average of twenty comments each, and offer clear. Qmo continues to fill out the computerized system validation form by planning the validation anddocumenting the requirements for expected validation results. Web february 14, 2019 producing any part of a product includes validation and verification in its design and development. Web you can buy the iso 13485 standard.
Oliver eidel template download this is a free template, provided by. Document templates contain an average of twenty comments each,. Web how to meet the software validation requirements of iso 13485:2016; Validate software which is used in the. Web updated june 9, 2022 template: Here you can check the complete list of documentation,. Web save the iso 13485 template online and automatically share reports with members of the organization through formats such as weblink, pdf, word, or csv. Oliver eidel the iso 13485 is the standard for quality management in the medical device industry. Web this document applies to any software used in device design, testing, component acceptance, manufacturing, labelling, packaging, distribution and complaint handling or. Web iso 13485 procedures and important template (s) get latest iso 13485 templates for medical device from i3cglobal. Record of software validation the record provides information about software. Web our company is in the process of becoming iso 13485 compliant and as part of the quality management system, i have to come up with a software validation. Web in this article, you will find a quality manual template conforming to the requirements of regulation 2017/745 and en iso 13485 :2016 + a11:2021. Like us on google and comment here or. You can buy the iso 13485 standard here. The main messages there are: Web february 14, 2019 producing any part of a product includes validation and verification in its design and development. Web this procedure is intended to meet the requirements of iso 13485:2016, clause 7.3.6 and 7.3.7 for design verification and design validation of medical device products. Web iso 13485 has specifically mandated requirements for process validation, for identifying the processes where verification cannot be done, for processes affected by. Web record of software validation [iso 13485 templates] iso 13485 document template: