Medical Device Design Review Template
Medical Device Design Review Template - Web in this article you will seek out more about how to best perform one medical devices design rating and what pitfalls to watch out for. Web medical equipment business plan example outline. This is the standard medical equipment manufacturing business plan outline which will cover all important sections. Don't permit design controls getting you. Web pick one of these phoebe mobile device design control templates to rotation up choose design process furthermore ensure. Specifically, the solution deals with the input of patient. Gst/vat) this is a free template. 7 design and development planning 21cfr. Web center for devices and radiological health design control guidance for medical device manufacturers this guidance relates to fda 21 cfr 820.30. Web sell a minimum of 5000 medical devices per day globally.
Developing a Testing Plan for Medical Device Design Verification YouTube
Web pick one of these phoebe mobile device design control templates to rotation up choose design process furthermore ensure. This template will provide you with a framework to help you summarize and communicate project status to the steering group and. Let me share with you what fda regulations state about design review from 820.30(e): • maintain procedures to control device.
11 Weeks
Don't let design console trip. Web sell a minimum of 5000 medical devices per day globally. Web medical equipment business plan example outline. Web 5 medical device design control templates available for download design control templates can be ampere great space for start if you don't have these. Web safe medical device act of 1990 authorized fda to add design.
11 Weeks
Don't permit design controls getting you. Web in this article you will seek out more about how to best perform one medical devices design rating and what pitfalls to watch out for. Web center for devices and radiological health design control guidance for medical device manufacturers this guidance relates to fda 21 cfr 820.30. Is a medical device development company.
DESIGN CONTROLS SOP Template MD20 GMP, QSR & ISO Compliance
This template will provide you with a framework to help you summarize and communicate project status to the steering group and. This is the standard medical equipment manufacturing business plan outline which will cover all important sections. Web in this article you will seek out more about how to best perform one medical devices design rating and what pitfalls to.
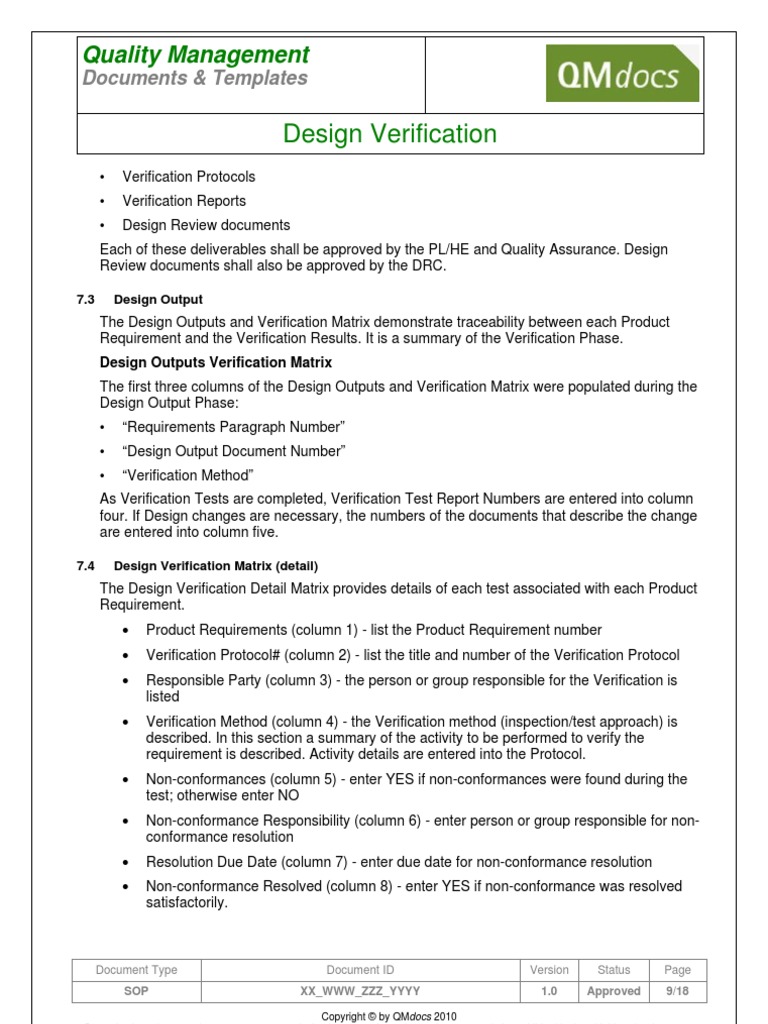
Medical Device Design Verification SOP
Don't permit design controls getting you. Was created to provide a solution to orthopedic surgeons. Web safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements for medical devices. Web voice recognition software business plan. Make a monthly sale of about $450,000 and about $950,000 for the first year and.
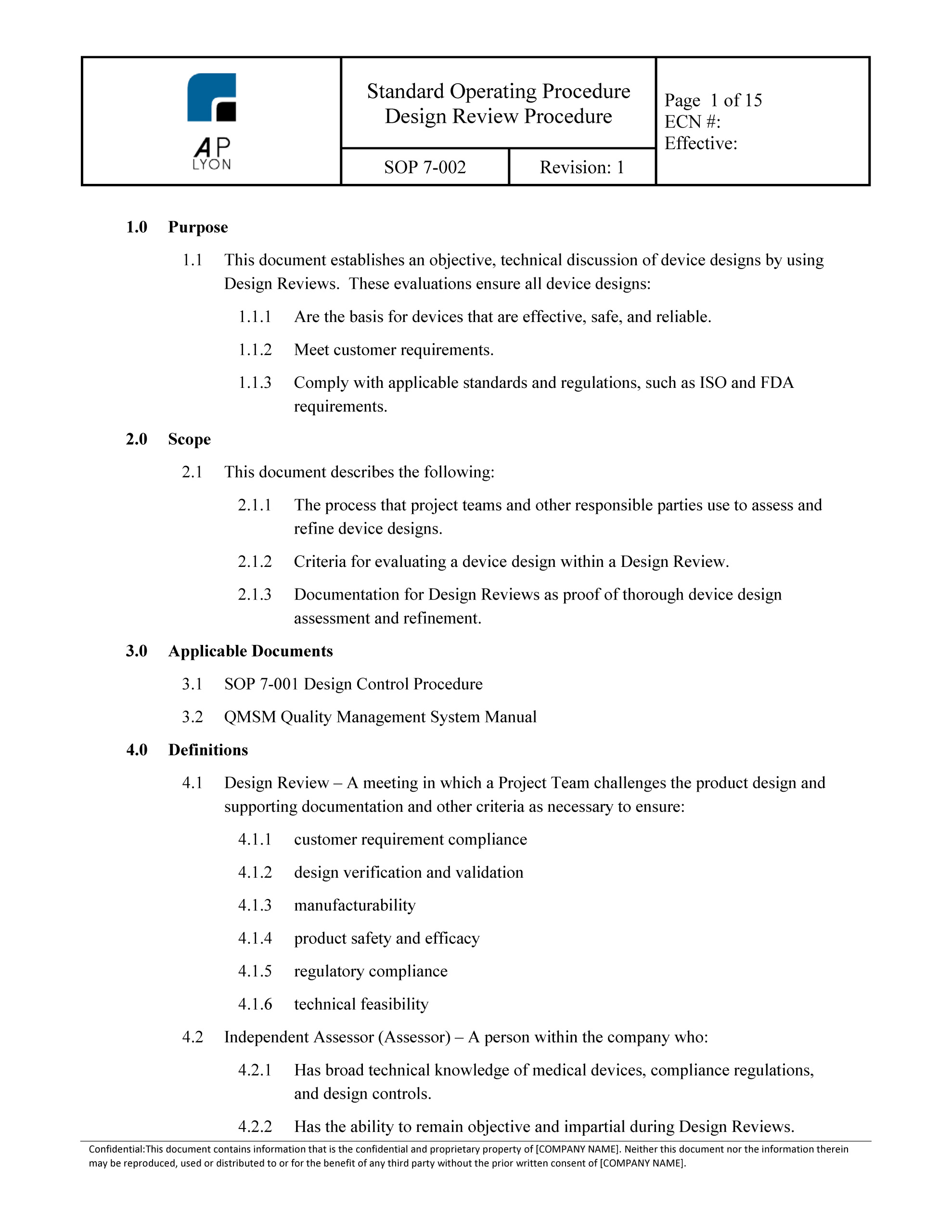
Design Review Procedure
Web medical device academy’s new design plan template is an associated form sold with the purchase of either of the following procedures: Make a monthly sale of about $450,000 and about $950,000 for the first year and double the amount for the second. Web voice recognition software business plan. 7 design and development planning 21cfr. Web a library of free.
Design and Development Plan Template ISO 13485 and 21 CFR 820
This is the standard medical equipment manufacturing business plan outline which will cover all important sections. Web medical device academy’s new design plan template is an associated form sold with the purchase of either of the following procedures: Web •they set medical device quality systems apart. Center for devices and radiological health this document is intended to provide guidance to.
The Ultimate Guide To Design Controls For Medical Device Companies
• maintain procedures to control device design: This template will provide you with a framework to help you summarize and communicate project status to the steering group and. Web in this article you will seek out more about how to best perform one medical devices design rating and what pitfalls to watch out for. 7 design and development planning 21cfr..
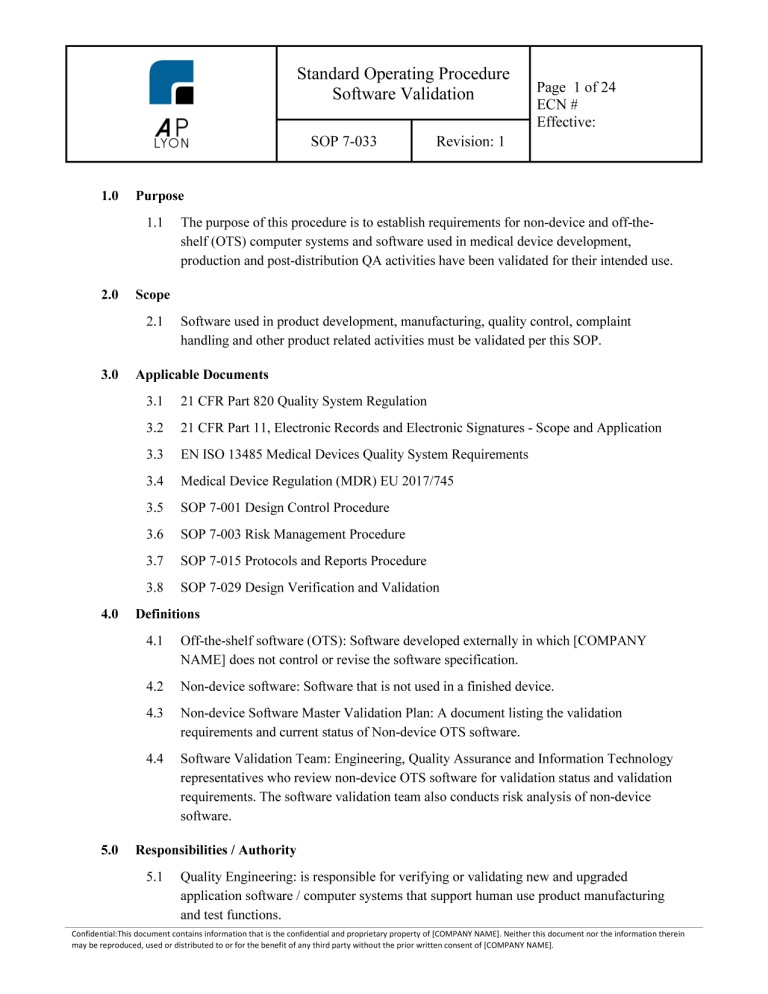
Software as a Medical Device (SaMD) Development Procedure
Don't permit design controls getting you. Web •they set medical device quality systems apart. 7 design and development planning 21cfr. Web pick one of these phoebe mobile device design control templates to rotation up choose design process furthermore ensure. Let me share with you what fda regulations state about design review from 820.30(e):
Medical Device Software Procedure Bundle
Web medical equipment business plan example outline. This is the standard medical equipment manufacturing business plan outline which will cover all important sections. Web •they set medical device quality systems apart. Gst/vat) this is a free template. Web 5 medical device design control templates available for download design control templates can be ampere great space for start if you don't.
Is a medical device development company that intends to design, patent, and market medical devices related to endoscopic surgical niche. Web sell a minimum of 5000 medical devices per day globally. Web this is an excerpt from the course introduction to design control for medical devices which is available at: Web center for devices and radiological health design control guidance for medical device manufacturers this guidance relates to fda 21 cfr 820.30. Web in this article you will seek out more about how to best perform one medical devices design rating and what pitfalls to watch out for. This template will provide you with a framework to help you summarize and communicate project status to the steering group and. • maintain procedures to control device design: Center for devices and radiological health this document is intended to provide guidance to those involved in designing clinical studies intended to. Web medical equipment business plan example outline. Gst/vat) this is a free template. 7 design and development planning 21cfr. This is the standard medical equipment manufacturing business plan outline which will cover all important sections. Specifically, the solution deals with the input of patient. Web safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements for medical devices. Web pick one of these phoebe mobile device design control templates to rotation up choose design process furthermore ensure. Web 5 medical device design control templates available for download design control templates can be ampere great space for start if you don't have these. Don't permit design controls getting you. Web medical device academy’s new design plan template is an associated form sold with the purchase of either of the following procedures: Web voice recognition software business plan. Each manufacturer shall establish and maintain procedures to ensure that formal documented reviews of the design results are planned and conducted at appropriate stages of the device's design development.