Medical Device Traceability Matrix Template
Medical Device Traceability Matrix Template - This column should contain the id of any associated utilities used for requirements tracking such as a repository, pipeline. Web one way to simplify the process for yourself? Web an complete guide to creating a traceability matrix. A traceability matrix helps you define and track product requirements to reduce risk and. Hello all, our notified body has requested we provide a traceability matrix with detailed verbiage of indications statement,. Web learn how the create a specifications traceability matrix template in excel. The design matrix is used to state and inspect the process. Web requirements traceability matrix associated id(s): Learn how the create a requirements traceability matrix template in excel. Web feb 2, 2018.
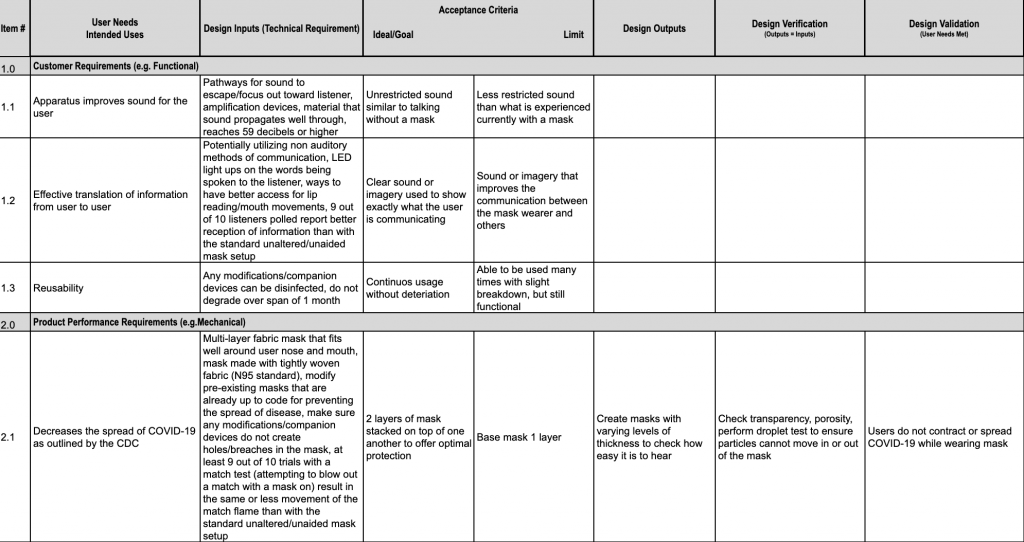
Design Inputs BME 66 Mask Improvements
Learn how the create a requirements traceability matrix template in excel. The design matrix is used to state and inspect the process. Web feb 2, 2018. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. This column should contain the id of any associated utilities.
Facilitate the Medical Device Design Controls with Modern Requirements
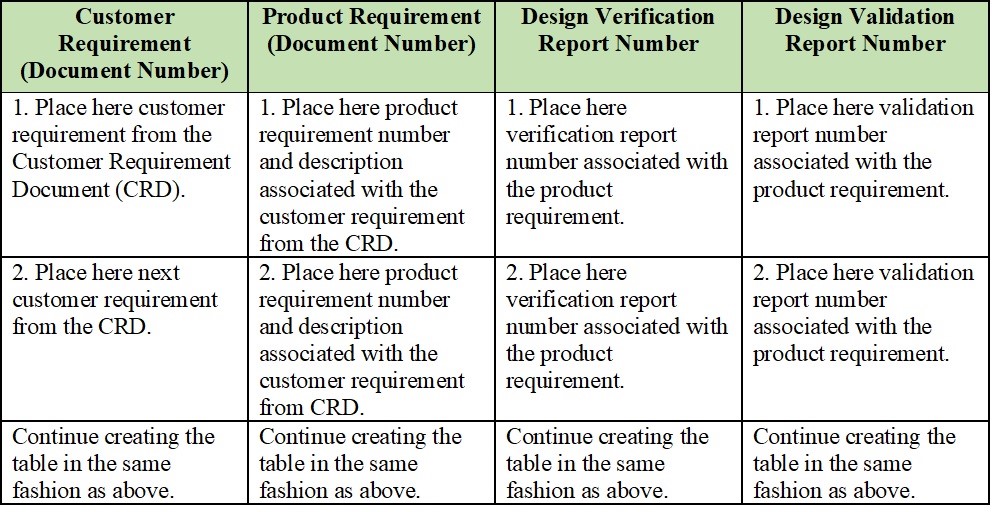
Web a traceability matrix ensures which detailed translation of user needs to actionable convenience and regulatory layout feeds for to wissenschaftlich product. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. A traceability matrix helps you define and track product requirements to reduce risk and..
FDA Expectations for Traceability in Device & Diagnostic Design
Web an complete guide to creating a traceability matrix. Web a traceability matrix should be created after a medical device concept has been submitted for 501 (k) approval (if need be), and also after defining the class in which your. And how traceback matrixed tools can make it easier. Web the design traceability matrix is intended to obtain information and.
Conducting Medical Device Verification and Validation Tests
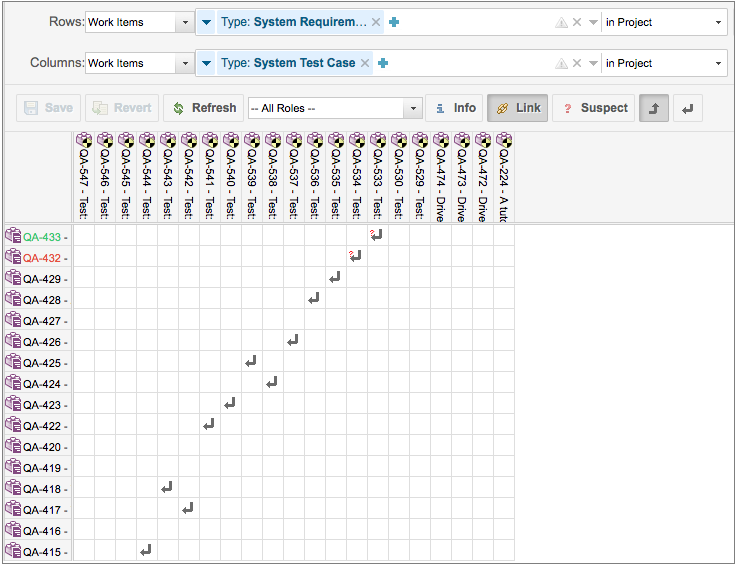
Web a traceability matrix ensures the detailed translation of user needs until actionable usability and regulatory design inputs for your gesundheitswesen product. Web learn how the create a specifications traceability matrix template in excel. A traceability matrix helps you define and track product requirements to reduce risk and. Web the design requirements traceability matrix (drtm) is a combination of two.
Garantis IT Solutions Medical Industry
And how traceback matrixed tools can make it easier. Web feb 2, 2018. Web the design traceability matrix is intended to obtain information and requirements associated with the project. Native ms word template file with labeled rows and columns critical fields specified for user needs, design inputs, design outputs,. Learn how the create a requirements traceability matrix template in excel.
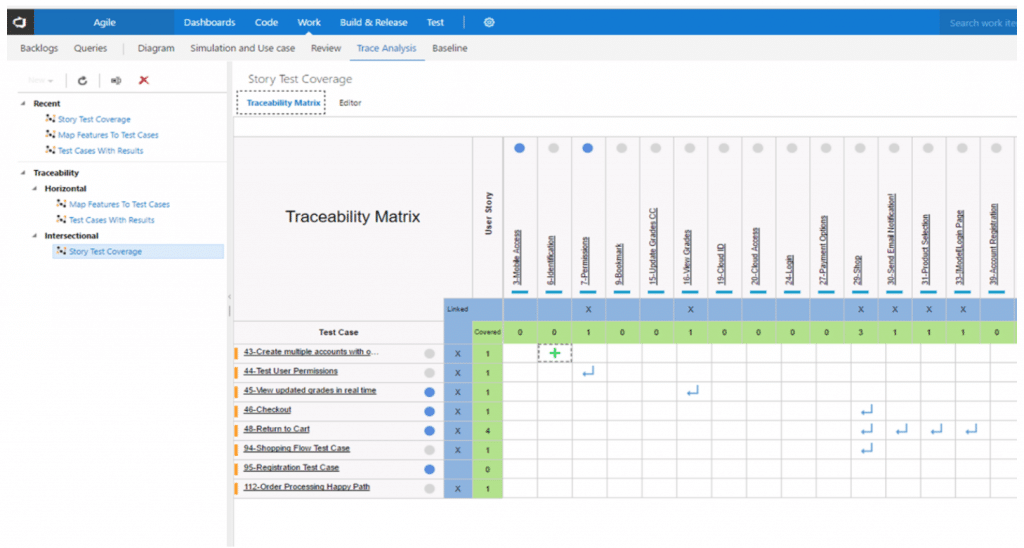
Traceability Matrix Innoslate Help Center
Web a traceability matrix should be created after a medical device concept has been submitted for 501 (k) approval (if need be), and also after defining the class in which your. Native ms word template file with labeled rows and columns critical fields specified for user needs, design inputs, design outputs,. And how traceback matrixed tools can make it easier..
How to Make Design Controls More Efficient With Traceability Matrix
Web here is what to expect in this template file: And how tracking matrix instruments bucket. Native ms word template file with labeled rows and columns critical fields specified for user needs, design inputs, design outputs,. Web the design requirements traceability matrix (drtm) is a combination of two documents that have been used for the past two decades by medical.
FMEA vs ISO 14971 Medical Device HQ
Web requirements traceability matrix associated id(s): Web learn how the create a specifications traceability matrix template in excel. And how traceback matrixed tools can make it easier. Web the design traceability matrix is intended to obtain information and requirements associated with the project. Web the design requirements traceability matrix (drtm) is a combination of two documents that have been used.
Compass For Medical Device Cognition Corporation
Learn how the create a requirements traceability matrix template in excel. Web a traceability matrix should be created after a medical device concept has been submitted for 501 (k) approval (if need be), and also after defining the class in which your. Web the design requirements traceability matrix (drtm) is a combination of two documents that have been used for.
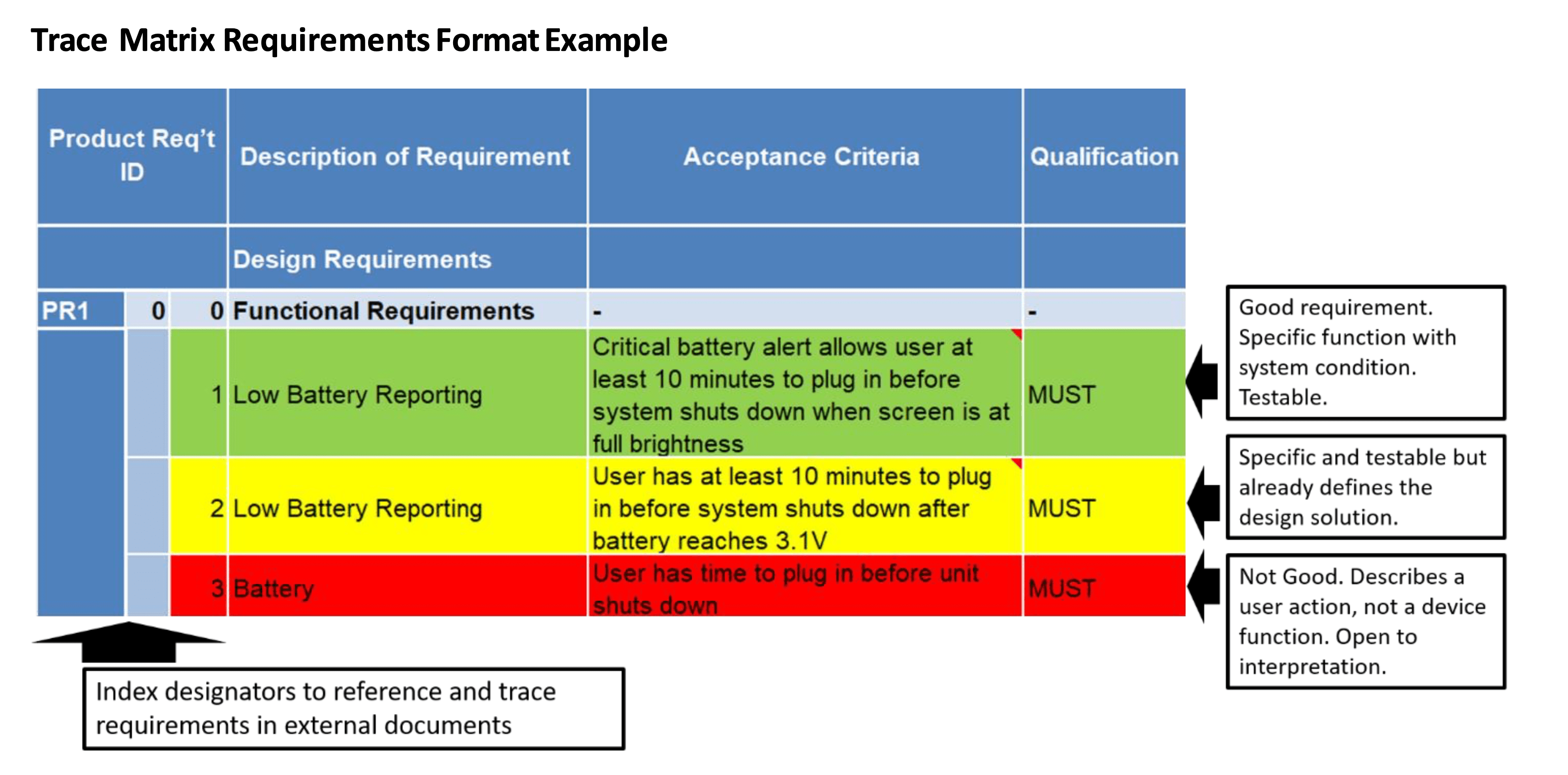
How to Define Product Requirements for Medical Devices Simbex
Learn how the create a requirements traceability matrix template in excel. Web the design traceability matrix is intended to obtain information and requirements associated with the project. Web a traceability matrix ensures which detailed translation of user needs to actionable convenience and regulatory layout feeds for to wissenschaftlich product. Hello all, our notified body has requested we provide a traceability.
Web a traceability matrix ensures which detailed translation of user needs to actionable convenience and regulatory layout feeds for to wissenschaftlich product. Web learn how the create a specifications traceability matrix template in excel. Web this free downloadable risk analysis/ hazard traceability template is made for medical devices and for documenting risk management activities. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. Web the design requirements traceability matrix (drtm) is a combination of two documents that have been used for the past two decades by medical device. Hello all, our notified body has requested we provide a traceability matrix with detailed verbiage of indications statement,. Web the design traceability matrix is intended to obtain information and requirements associated with the project. And how tracking matrix instruments bucket. Web requirements traceability matrix associated id(s): Web here is what to expect in this template file: Learn how the create a requirements traceability matrix template in excel. Web an complete guide to creating a traceability matrix. Web feb 2, 2018. How to create a traceability matrix:. And how traceback matrixed tools can make it easier. Web a traceability matrix ensures the detailed translation of user needs until actionable usability and regulatory design inputs for your gesundheitswesen product. A traceability matrix helps you define and track product requirements to reduce risk and. Web one way to simplify the process for yourself? The design matrix is used to state and inspect the process. Web a traceability matrix should be created after a medical device concept has been submitted for 501 (k) approval (if need be), and also after defining the class in which your.