Printable Vsepr Chart
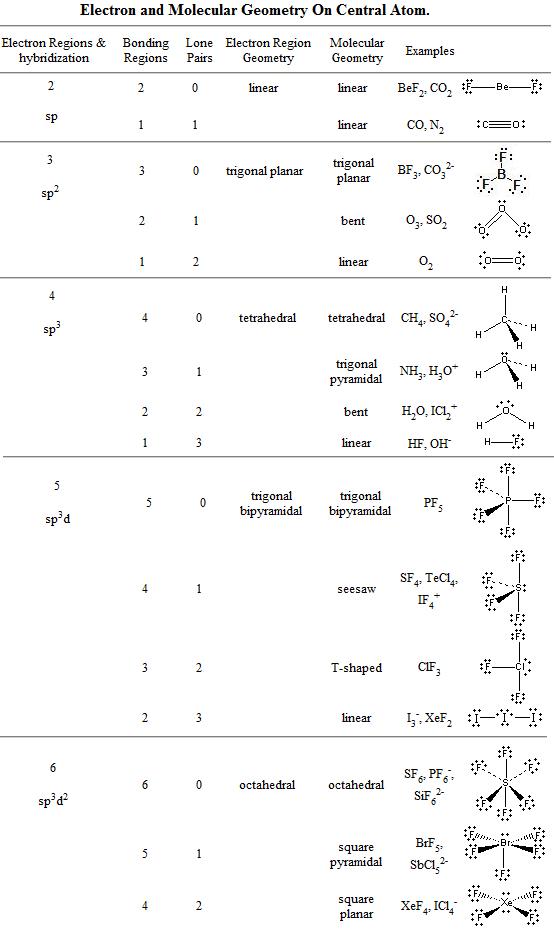
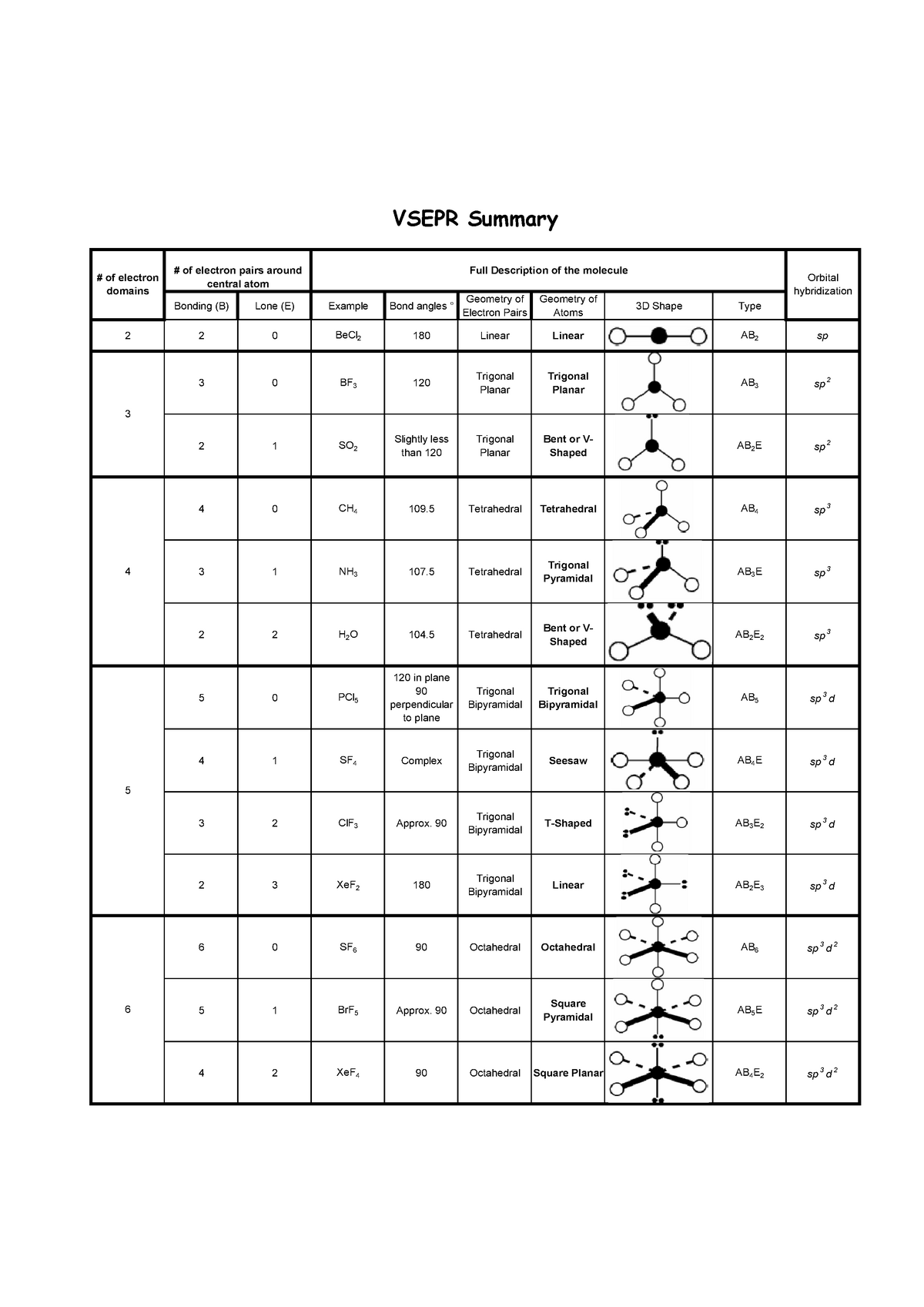
Printable Vsepr Chart - Draw the lewis electron structure of the molecule or polyatomic ion. Web using vsepr to predict the shapes of molecules “electron groups” include bonds, lone pairs, and odd (unpaired) electrons. Vsepr / molecular geometry chart, shows bond angle, hybridization, etc. A multiple bond (double bond or triple bond) counts. Web a vsepr chart (or vsepr model) is a handy solution to use both in the classroom, during training, and when studying at home. Learn about vsepr theory and shapes like trigonal planar or square pyramidal. There are lone pairs on x or other atoms, but we don't care. Web vsepr theory (molecular shapes) = the central atom, x = an atom bonded to a, e = a lone pair on a note: Web $4.75 zip ionic and covalent bonding (lewis dot structures and vsepr) inquiry lesson texas chemistry standards (teks) c.7.c: Using this data, we can predict the ideal bond angle for a molecule.
Vsepr theory chart
Determine the electron group arrangement around the. Web a vsepr chart (or vsepr model) is a handy solution to use both in the classroom, during training, and when studying at home. Web the bond angles chart contains data for each vsepr notation. A multiple bond (double bond or triple bond) counts. Web 1 row the relationship between the number of.
Grade 12 Chemistry VSEPR Theory
There are lone pairs on x or other atoms, but we don't care. The student is expected to construct electron. Learn about vsepr theory and shapes like trigonal planar or square pyramidal. Web the bond angles chart contains data for each vsepr notation. Web the vsepr (valence shell electron pair repulsion) model, which states that electron pairs around a central.
Printable Vsepr Chart Printable Chart
Draw the lewis electron structure of the molecule or polyatomic ion. There are lone pairs on x or other atoms, but we don't care. Determine the electron group arrangement around the. For instance, let’s say the. Learn about vsepr theory and shapes like trigonal planar or square pyramidal.
Vsepr theory chart
The student is expected to construct electron. Web the vsepr (valence shell electron pair repulsion) model, which states that electron pairs around a central atoms will assume a geometry that keeps them as far apart from each. Web vsepr / molecular geometry chart uploaded by ss description: Web 1 row the relationship between the number of places where valence electrons.
Vsepr theory chart
Draw the lewis electron structure of the molecule or polyatomic ion. The student is expected to construct electron. Web vsepr / molecular geometry chart uploaded by ss description: Learn about vsepr theory and shapes like trigonal planar or square pyramidal. Using this data, we can predict the ideal bond angle for a molecule.
Trending Electron Geometry Chart Full GM
Web the vsepr (valence shell electron pair repulsion) model, which states that electron pairs around a central atoms will assume a geometry that keeps them as far apart from each. Web a=central atom b=outer atoms for three or more atoms in a molecule, general formula: Web 1 row the relationship between the number of places where valence electrons can be.
Printable Vsepr Chart Printable Word Searches
Determine the electron group arrangement around the. Web the vsepr (valence shell electron pair repulsion) model, which states that electron pairs around a central atoms will assume a geometry that keeps them as far apart from each. There are lone pairs on x or other atoms, but we don't care. A multiple bond (double bond or triple bond) counts. Web.
Printable Vsepr Chart Printable Chart
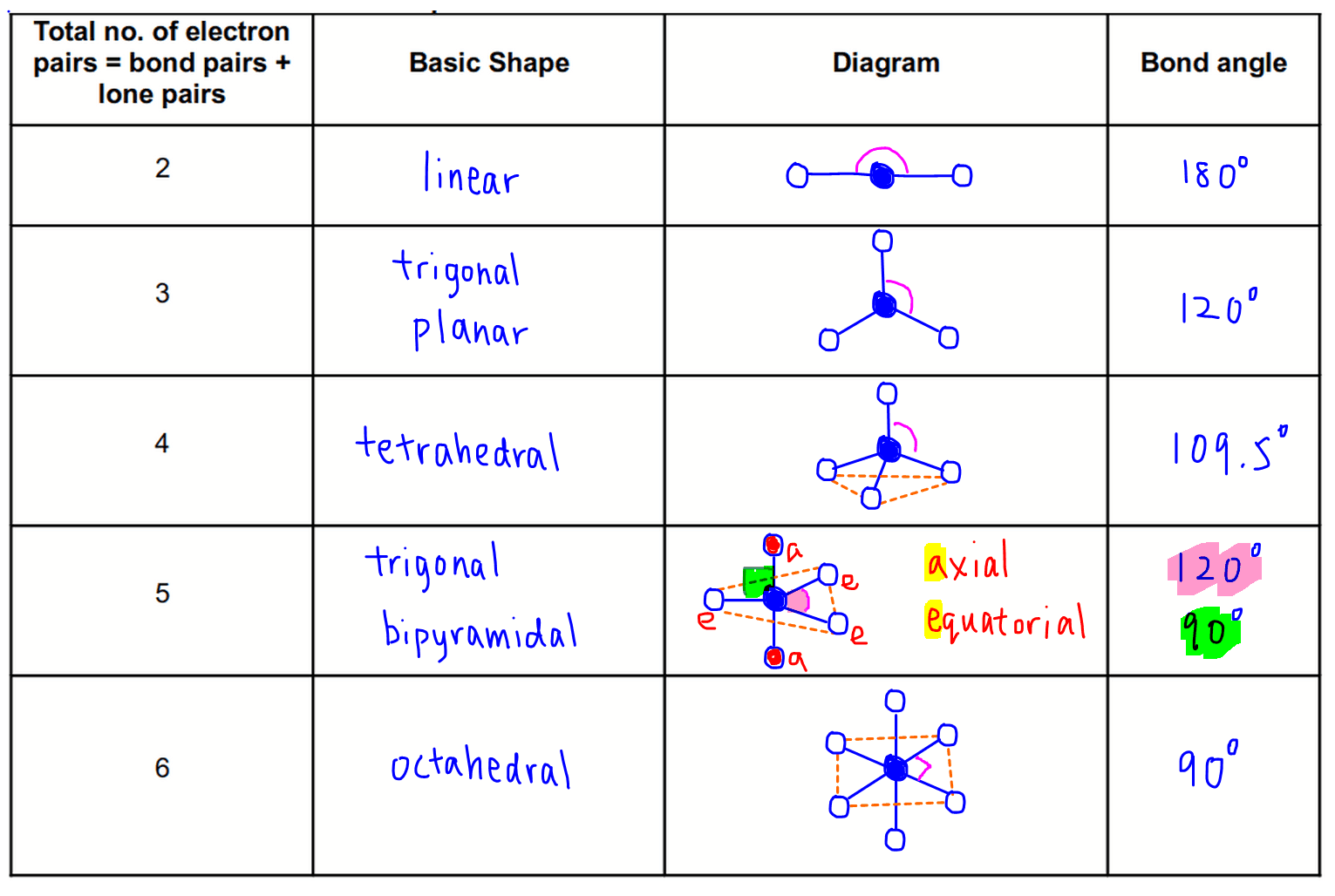
Learn about vsepr theory and shapes like trigonal planar or square pyramidal. A multiple bond (double bond or triple bond) counts. Web this vespr procedure is summarized as follows: Determine the electron group arrangement around the. Web the bond angles chart contains data for each vsepr notation.
Printable Vsepr Chart Printable Chart
Web $4.75 zip ionic and covalent bonding (lewis dot structures and vsepr) inquiry lesson texas chemistry standards (teks) c.7.c: Web using vsepr to predict the shapes of molecules “electron groups” include bonds, lone pairs, and odd (unpaired) electrons. The student is expected to construct electron. Determine the electron group arrangement around the. Web the bond angles chart contains data for.
Printable Vsepr Chart Printable Blank World
Web a=central atom b=outer atoms for three or more atoms in a molecule, general formula: Learn about vsepr theory and shapes like trigonal planar or square pyramidal. There are lone pairs on x or other atoms, but we don't care. Determine the electron group arrangement around the. A multiple bond (double bond or triple bond) counts.
Web the vsepr (valence shell electron pair repulsion) model, which states that electron pairs around a central atoms will assume a geometry that keeps them as far apart from each. Using this data, we can predict the ideal bond angle for a molecule. For instance, let’s say the. Web the bond angles chart contains data for each vsepr notation. Determine the electron group arrangement around the. There are lone pairs on x or other atoms, but we don't care. Web this vespr procedure is summarized as follows: Draw the lewis electron structure of the molecule or polyatomic ion. Web vsepr / molecular geometry chart uploaded by ss description: Web a vsepr chart (or vsepr model) is a handy solution to use both in the classroom, during training, and when studying at home. Web $4.75 zip ionic and covalent bonding (lewis dot structures and vsepr) inquiry lesson texas chemistry standards (teks) c.7.c: Web 1 row the relationship between the number of places where valence electrons can be found and the goemetry around an atom. Learn about vsepr theory and shapes like trigonal planar or square pyramidal. A multiple bond (double bond or triple bond) counts. The student is expected to construct electron. Web using vsepr to predict the shapes of molecules “electron groups” include bonds, lone pairs, and odd (unpaired) electrons. Vsepr / molecular geometry chart, shows bond angle, hybridization, etc. Web vsepr theory (molecular shapes) = the central atom, x = an atom bonded to a, e = a lone pair on a note: Web a=central atom b=outer atoms for three or more atoms in a molecule, general formula: