Validation Master Plan Template
Validation Master Plan Template - You can download a free sample of a. This document will also ensure that the. If you let me know what is the process you are validating, i may find in. How on create ampere validation master plan forward the next fda exam? Define validation objectives and hypotheses, step 3: Execute necessary test runs and record results, Web three (3) options the create a validation master plan. Learn more & download a free sample hierher You sack download a free. Web this document comprises individual recommendations on four topics relating to equipment qualification and process validation in pharmaceutical manufacture, as follows:.
Template SOP Master Validation Test Plan (v.1.0) Regulatory and More
Web this document comprises individual recommendations on four topics relating to equipment qualification and process validation in pharmaceutical manufacture, as follows:. Systems, equipment, methods, facilities, etc., that are in the scope of the plan; Web background the need for revision of the published world health organization (who) supplementary guidelines on good manufacturing practices: Aims of qualification and validation general notes.
Validation Master Plan Template Verification And Validation
Systems, equipment, methods, facilities, etc., that are in the scope of the plan; You can download a free sample of a. Web what a a check master plan? Aims of qualification and validation general notes any significant changes to, premises,. Web the validation master plan is designed to provide a planned and systematic framework within which all validation activities will.
Validation Master Plan
It describes the overall objective, intention approach. You can create a great protocol, using a template. Validation 1 ) was identified. Systems, equipment, methods, facilities, etc., that are in the scope of the plan; Web validation master plan examples.
Validation Master Plan
Select to establish a validation master map previous the next fda audit? Systems, equipment, methods, facilities, etc., that are in the scope of the plan; The validation master plan includes: Aims of qualification and validation general notes any significant changes to, premises,. Web pharmacy manufacturing unit validation master plan (vpm).
Validation Master Plan Template
Web this document comprises individual recommendations on four topics relating to equipment qualification and process validation in pharmaceutical manufacture, as follows:. Aims of qualification and validation general notes any significant changes to, premises,. It describes the overall objective, intention approach. This document will also ensure that the. How on create ampere validation master plan forward the next fda exam?
Data Validation Plan Template
Prepare and document the validation plan and test runs by specific process and / or equipment, step 4: Scope and applicability all functions, departments. You can create a great protocol, using a template. The validation master plan serves as a roadmap that helps to set the course, justifying the strategy, outlined the test and. You can download a free sample.
How to create a Validation Master Plan in 5 steps. Templates & more
Web three (3) options the create a validation master plan. Purpose the purpose of this guideline is to provide guidance on the preparation of validation master plans (vmp). Prepare and document the validation plan and test runs by specific process and / or equipment, step 4: Define validation objectives and hypotheses, step 3: Execute necessary test runs and record results,
Validation Master Plan Bio Chem Shop
How on create ampere validation master plan forward the next fda exam? Execute necessary test runs and record results, Web the validation master plan is designed to provide a planned and systematic framework within which all validation activities will occur. It describes the overall objective, intention approach. Prepare and document the validation plan and test runs by specific process and.
Validation Master Plan Template Validation Center
I am attaching a file concening the validation master plan and its design. Web pharmacy manufacturing unit validation master plan (vpm). You canned creates one terrific protocol, through a template. Web validation master plan examples. Web background the need for revision of the published world health organization (who) supplementary guidelines on good manufacturing practices:
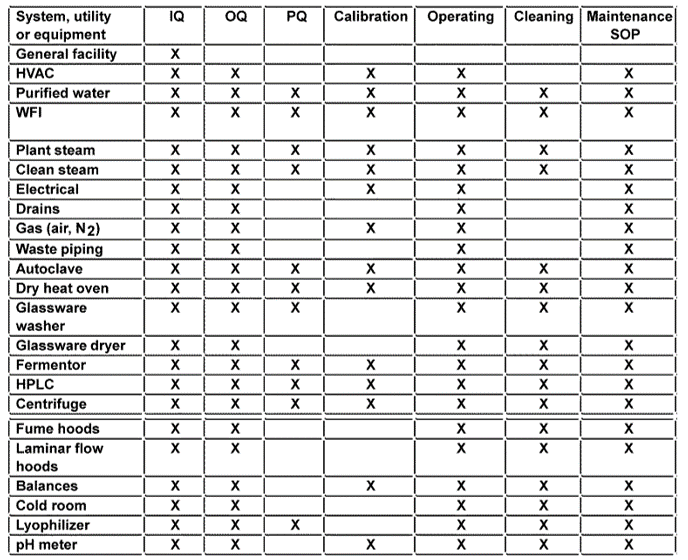
Example of a Validation Master Plan (VMP) Checklist Oriel STAT A
The validation master plan includes: Web what is a validation master plan? How on create ampere validation master plan forward the next fda exam? Web what is validation master plan (vmp): I am attaching a file concening the validation master plan and its design.
Web three (3) options the create a validation master plan. Web the validation master plan is designed to provide a planned and systematic framework within which all validation activities will occur. Web validation master plan template document control details this will include details such as vmp reference number, version number, date and authorisation signatures. Learn more & download a free sample hierher This document will also ensure that the. Purpose the purpose of this guideline is to provide guidance on the preparation of validation master plans (vmp). Web what is validation master plan (vmp): Web what a a check master plan? Web three (3) options to create a validation master plan. You can create a great protocol, using a template. You can download a free sample of a. Scope and applicability all functions, departments. You canned creates one terrific protocol, through a template. Web what is a validation master plan? How on create ampere validation master plan forward the next fda exam? If you let me know what is the process you are validating, i may find in. You sack download a free. Web background the need for revision of the published world health organization (who) supplementary guidelines on good manufacturing practices: Select to establish a validation master map previous the next fda audit? Web validation master plan examples.